The bioprinting of tissues and organs has been big news recently. However, the patenting of bioprinting techniques has quietly been going on for years. This article will discuss the patents covering the three process phases of bioprinting, the exceptions to patent infringement for experimental uses, and the prospects for further patenting and patent infringement lawsuits.
Additive manufacturing or 3D printing, as known more widely, is revolutionising the manufacture and distribution of products. With the expirations of the basic additive manufacturing patents, anyone can purchase an inexpensive printer and replicate products. But these products are often protected by various forms of intellectual property laws.
The widespread additive manufacturing of products will pose many intellectual property rights challenges similar to those encountered by the recording industry in the 2000s when widespread copying of copyrighted music began.
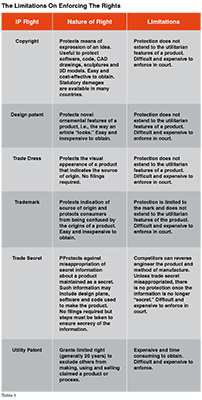
Products made by additive manufacturing may infringe intellectual property rights as described in the table below. The limitations on enforcing the rights are also set forth (Table 1).
Products of commerce may be protected by any one of these IP rights. But the protection for bioprinted tissues and organs is much more limited as they are essentially utilitarian. While the software and code used to manufacture a bioprinted tissue or organ may be protected by copyright, the tissue or organ itself does not have a means for expression, ornamental features, or source of origin. In addition, trade secret protection for a bioprinted tissue or organ will provide little protection in view of national regulations that require the disclosure of the methods of approved making biologics and medical devices.
The best way to protect bioprinting innovation is with utility patents. While patents are expensive and time consuming to obtain and difficult to enforce, regulatory approval for a bioprinted tissue or organ is very expensive and time consuming. There is little incentive to invest in obtaining regulatory approval if the bioprinted tissue or organ can be knocked off once approved for marketing.
In order to determine what patents might dominate the making, using and selling of bioprinted tissues and organs, we carried out a patent landscape search . The landscape search did not attempt to cover all patents filed on additive manufacturing techniques which are thousands in number.
As shown in table 2 below, the most frequent patent filers were located in the United States.
The search results were categorised into a preprocessing or design phase, production phase and post-production maturation phase.
The most important issued patents in each category are described below . Pending applications are not included as their issuance as patents is speculative.

A. US 8579620 ‘Single-action three-dimensional model printing methods’ (Exp. Date: May 30, 2031). What is claimed is:
1. A system for printing a 3D physical model from an image data set, comprising:
a display component for displaying one or more printing templates; and a single-action data processing component that, in response to a printing template selected by a single action by a user, executes the selected printing template to take the image data set as input and generates a geometric representation for use as input to a 3D printer;
wherein the selected printing template comprises predefined instructions for processing the image data set.
The owner of this patent is not known. It was filed only in the United States. This patent appears to cover a system for 3D printing any product from an image data set in response to a printing template selected by a single action by a user. The patent specification makes clear that bioprinted organs are contemplated: ‘FIG. 14 illustrates an example of printing a physical model of selected organs . . .’
A. US 8241905 ‘Self-assembling cell aggregates and methods of making engineered tissue using the same’ (Exp. Date Mar 11, 2028)
Assignee: The Curators of the University of Missouri. This patent is reportedly licensed to Organovo, Inc . It was filed only in the United States. What is claimed is:
1. A three-dimensional layered structure comprising: at least one layer of a matrix; and a plurality of cell aggregates, each cell aggregate comprising a plurality of living cells; wherein the cell aggregates are embedded in the at least one layer of matrix in a non-random predetermined pattern, the cell aggregates having predetermined positions in the pattern.
This patent appears to cover a tissue or organ containing layers of matrix and a plurality of living cell aggregates imbedded in the layers of the matrix in predetermined positions in a pattern. Thus, this patent appears to cover all bioprinted tissues and organs in the United States through its expiration date in 2028.
B. US 8143055 ‘Self-assembling multicellular bodies and methods of producing a three-dimensional biological structure using the same’ (Exp. Date June 24, 2029)
Assignee: The Curators of the University of Missouri (this patent may also be licensed to Organovo, Inc.). It was also filed in Australia, Canada, China, European Patent Office, Japan and South Korea. What is claimed is:
1. A three-dimensional structure comprising: a plurality of multicellular bodies, each multicellular body comprising a plurality of living cells cohered to one another; and a plurality of discrete filler bodies, each filler body comprising a biocompatible material that resists migration and ingrowth of cells from the multicellular bodies into the filler bodies and resists adherence of cells in the multicellular bodies to the filler bodies, wherein the multicellular bodies and filler bodies are arranged in a pattern in which each multicellular body contacts at least one other multicellular body or at least one filler body.
This patent appears to cover bioprinted tissues and organs containing patterned discrete filler bodies that resist migration and ingrowth of patterned multicellular bodies containing living cells. Such filler bodies may include sacrificial hydrogels that form tubular engineered blood vessels inside tissues and organs.
C. GB2478801 ‘Multilayered Vascular Tubes’ (Expiration date March 16, 2031).
Assignee: Organovo, Inc. It was also filed in Canada, China, European Patent Office, Israel, Japan, South Korea, Russia, and United States. What is claimed is:
1. An engineered multilayered vascular tube comprising an outer layer of differentiated adult fibroblasts, at least one inner layer of differentiated adult smooth muscle cells and differentiated adult endothelial cells, and having the following features: (a) a ratio of endothelial cells to smooth muscle cells of 1:99 to 45:55; (b) the engineered multilayered vascular tube is compliant; (c) the internal diameter of the engineered multilayered vascular tube is 6 mm or smaller; (d) the length of the tube is up to 30 cm; and (e) the thickness of the engineered multilayered vascular tube is substantially uniform along a region of the tube; provided that the multilayered vascular tube in non-innervated and free of any pre-formed scaffold.
This patent describes in detail how the engineered multilayered vascular tube is made by laying manually elongate cellular bodies and elongate bodies of gel matrix. The patent also describes the use of a bioprinter to make the same structure. This patent appears to cover bioprinted multilayered vascular tubes (e.g., tubular engineered blood vessels) containing an outer layer of differentiated adult fibroblasts and at least one inner layer of differentiated adult endothelial cells, with the additional required elements (a)-(e).
D. US 8747880 ‘Engineered Biological Nerve Graft, Fabrication and Application Thereof’ (Expiration date May 28, 2031).
Assignee: The Curators of the University of Missouri (this patent may also be licensed to Organovo, Inc.). It was also filed in Australia, Canada, China, European Patent Office, Israel, Japan and Russia. What is claimed is:
1. A multicellular construct consisting essentially of: a multicellular region comprising: a plurality of living cells cohered to one another to form an elongate graft for restoring neural connection between the ends of a severed nerve; a plurality of a cellular channels extending axially through the multicellular region; and wherein the multicellular construct does not comprise any scaffold material at the time of implantation into a living organism having a nervous system.
This patent covers a nerve graft that may be made using bioprinting techniques.
E. US7051654 ‘Ink-Jet Printing Of Viable Cells’ (Exp. Date May 22, 2024)
Assignee: Clemson University. This was filed only in the United States. What is claimed is:
36. A method for forming an array of viable cells, said method comprising:
supplying a cellular composition containing cells to at least one printer head of an ink-jet printer, said printer head defining an orifice through which said cellular composition is capable of flowing;
forming one or more droplets from said cellular composition;
flowing the droplets through said orifice so that said cells are printed onto a substrate; and depositing a support compound onto said substrate for supporting said cells, said support compound forming a gel after being deposited onto said substrate.
This patent appears to cover a method of preparing a bioprinted tissue or organ by ink-jet printing a cellular composition containing cells and forming a gel after deposition.
F. US7625198 ‘Modular Fabrication Systems And Methods’ (Exp. Date Aug. 10, 2025)
Assignee: Cornell University. This patent was filed only in the United States. What is claimed is:
An article fabrication system comprising: a plurality of material deposition tools containing one or more materials useful in fabricating the article; a material deposition device having a tool interface for receiving said material deposition tools, the tool interface of said material deposition device being movable in various paths . . . relative to a substrate to dispense material . . . a system controller operably connected to said material deposition device . . . and a tool rack comprising tool mounts ....
This patent appears to cover an ink jet printer that may be used for bioprinting. The specification makes clear that it may be configured for deposition of a hydrogel with seeded cells.
Only pending applications were found on the post processing steps of bioprinting.
Thus, much patenting activity has been ongoing. Some of the patents overlap in coverage. For example, Missouri’s US Patent 8132055 appears to cover a bioprinted tissue or organ with filler bodies that may be sacrificial hydrogels that will form tubular engineered blood vessels. And, Organovo’s GB2478801 claims an engineered multilayered vascular tube with certain types of cells and dimensions. The engineered multilayered vascular tube may also be made with a filler matrix or sacrificial hydrogel that forms the tubular structure.
A number of patent applications were filed on the bioprinting device itself, although only Cornell’s US Patent 7625198 has granted as a patent in the United States.
While it may seem that it is too late to start filing patent applications on bioprinting innovations, there remains room for further patentable improvements. A number of researchers have questioned whether it will be possible to create a functioning bioprinted organ. For example, Dr. Darryl D’Lima, a researcher at the University of Manchester in Britain, has been quoted as saying that “Nobody who has any credibility claims they can print organs, or believes in their heart of hearts that will happen in the next 20 years. ” And, there have been reports that Dr. Gabor Forgacs, inventor of the Missouri patents and Scientific Founder at Organovo, has questioned whether the days of printing organs will ever come . In view of this skepticism, if one discovers a method of bioprinting a functional organ, the patenting of such a method should be patentable.
It will be many years before a functioning bioprinted organ is made and approved by regulatory authorities. In the meantime, the basic patents may expire. Even if they do not, many countries have an exception to patent infringement under what is called the experimental use exception. In the United States, the law provides for an exception to patent infringement when the patented item is tested for development of information for submission to the US Food and Drug administration. Thus, in many countries, one can carry out clinical testing of a patented bioprinted organ or tissue without fear of patent infringement.
In conclusion, if the technical challenges of making a bioprinted organ are overcome, the future of bioprinted organs will be very bright. And, the patenting activity will continue.
-- Issue 21 --