




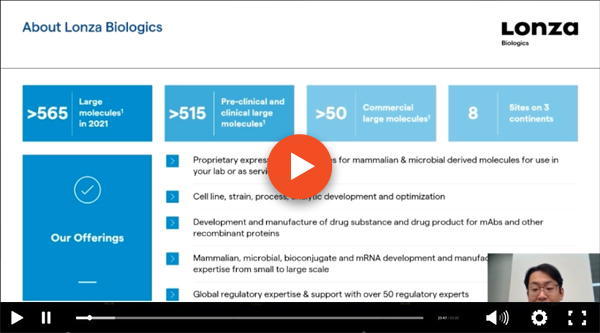
Advancing your drug candidate from late-stage discovery into the clinic is one of the most critical steps in development. To maximize your chances of success, it is essential to de-risk your candidate(s) as soon as possible. While manufacturability and immunosafety issues can threaten your molecule’s path to the clinic, they can be predicted and managed very early in the development journey based on the candidate’ sequence and structure.
Join our expert Dr. Yvette Stallwood, Head of Early Development Services at Lonza Biologics to explore the challenges and key considerations to de-risk your biotherapeutic(s). Learn how in silico and in vitro design and optimization tools allow you to assess and mitigate risks early and ultimately increase the likelihood of a successful First-in-Human campaign.
Participants will learn about
Who should Attend
Register to get Instant Video access

Dr. Yvette Stallwood Ph.D
Head of Early
Development Services
Yvette Stallwood completed her PhD at the University of Birmingham (UK) and has a background in Virology, Cell & Molecular Biology. She joined Lonza in 2007, initially leading the cell and molecular biology expression group in the Applied Protein Services department and is currently Head of Early Development Services and Head of Cambridge site. The Early Development Services team are focussed on the development and provision of services to support the development of new biotherapeutic proteins and vaccines with a particular focus on immunogenicity, manufacturability and protein expression.