


Since the year of 2000, there’re 13 ADCs on the market and 9 of them were approved in the last three years. The enthusiasm of pharmaceutical and biotechnology companies for the research and development of ADC has been increasing. How to quickly complete the early development of ADC through differentiation strategy has become an important link for companies to focus on competition. WuXi XDC has established a one-stop discovery service platform, covering from protein/antibody generation, linker-payload synthesis, bioconjugation, in vitro/in vivo functional studies and developability study, for bioconjugate drugs to meet the early research needs of the customers.
Scientists
Bioprocess Engineers
Bioproduction Engineers
Process Development Engineers
Process Development Managers
Consultants R&D Scientists
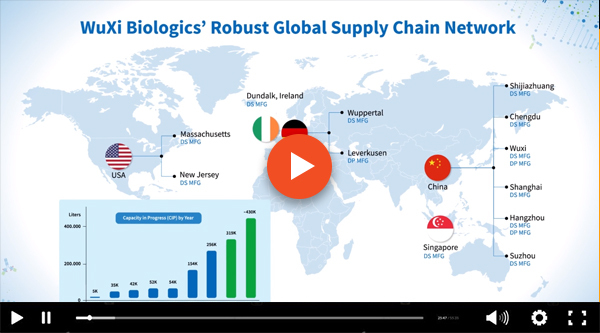
Register for free to get video access

Dr. Jiawei Lu
Director of Bioconjugates, Discovery Services, WuXi XDC
Dr. Jiawei Lu , director of Bioconjugates Discovery Services from WuXi XDC, has ten years of experiences in bioconjugation. Now he is responsible of building a first-class discovery services empower platform of conjugated drugs. His team has extensive experiences in conjugation, purification, and characterization of various types of conjugates, including ADC, PEGylated protein, bispecific antibodies, Antibody-Oligonucleotide conjugates, Antibody-Chelator conjugates, 2nd antibody, etc. In the past 8 years, his team has built long-term collaboration with numerous customers and has successfully advanced plenty of lead molecules from discovery stage to CMC stage.