Bioreactor performance for mammalian cell culture has been automated and improved with better soft-sensor inline analytics and model predictive process control. With simple customisation of CHO cell models using a novel software tool, precise and stable glucose feed-regulation specific to user cell lines and media requirements is enabled. This fully-integrated hardware and software system provides unprecedented custom automation and reproducibility for biologic product performance in CHO cell culture at the lab-scale.
In the quest for improved quality and productivity in drug manufacturing, the industry is moving toward increasing use of bioreactor systems with realtime integrated monitoring and advanced analytics that have the potential to enable automation, drive performance and improve data-rich quality control. However, there exist multiple options in sensors and technologies for monitoring important cell culture variables or Critical Process Parameters (CPP). Furthermore, cell culture vessel configurations can be disposable Single-use Bioreactors (SUBs), glass or even stainless-steel. They can be stirredtank in design, rocking platform bags or perfusion configurations. The sensors and monitoring technologies selected for these configurations need to be suitably designed and compatible with the bioreactor architecture. When properly selected, the Process Analytical Technologies (PAT) provide not only analytical insights into ongoing bioprocesses, but can be leveraged for real-time control and automation; especially in fed-batch or continuous culture.
Automation is a key trend driving improvements in manufacturing; especially for production of biologics in the biopharmaceutical industry. One big challenge is the integration of systems for effective automation. For example, recent uses of in-line sensors in mammalian cell culture can be used to monitor biomass production, key media nutrients, or metabolism indicators. Spectrophotometric techniques such as Near-infrared (NIR) and RAMAN technology have been employed to quantitate and correlate spectroscopic data with precise chemical signatures. As a result, the monitoring of glucose concentrations and lactate in real-time are becoming more common, reducing the need for off-line analysis and enabling the automated delivery of media supplements as required.
Comparing the capability of RAMAN and FT-NIR for spectroscopic applications in cell culture is frequently a debate among researchers. The ability to spectroscopically identify and differentiate cell culture media components is primarily affected by considerations of bandwidth, sensitivity, interference and software. And the latter (software), is quite capable of having a sizable impact on the prior three mentioned parameters as described in the examples below.
For instance, in bioreactor culture, two issues frequently cited and considered limitations of FT-NIR included baseline data variation due to cell density dependent optical scattering and interference by water in glucose detection. We have convincingly addressed the first issue largely by digital noise suppression software improvements. Whereas the second issue of signal interference by water or other molecules overlapping with glucose bond absorption profiles was reduced by the proper selection of, and integration of multiple wavelength micro-scan data to provide a more specific footprint of reliable molecule-specific bond signatures. Both of these NIR limitations were thus overcome by innovative data use and novel software approaches. Consequently, the determination of glucose concentration in cell culture media with FT-NIR can now be robustly correlated with actual reference concentrations or off-line cell culture glucose measurements using bench top chemical analyzers.
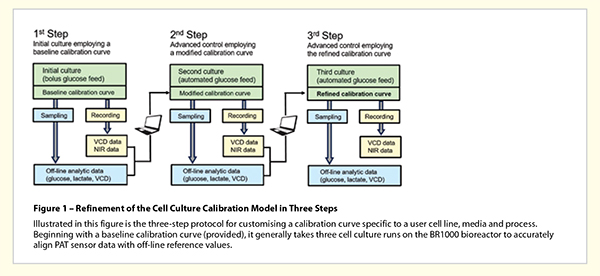
The derivation of cell models and the proper selection of micro-scan data for a particular cell line and cell culture medium are fairly complex tasks requiring significant resource time and effort to achieve the proper calibration. And although a universal cell model for any expression system would seem to be ideal, it is not an effective viable option. To this end, we have settled on a precondition and a calibration approach to simplify the alignment of NIR data with user-specific cells and media. The precondition is to focus on a specific cell type so that deviations from the standard model are minimised. And the calibration approach is one that leverages a baseline calibration curve to provide a suitable starting point for subsequent user data-derived refinements.
By limiting the developed FT-NIR application to specifically CHO cells, we were able to derive an effective lap-top software tool that uses a ‘datadriven’ model refinement algorithm. The tool enables the custom calibration of FT-NIR data to any CHO-cell based user expression system by smartly adjusting spectroscopic micro-scan data. It has been our experience that starting with the pre-set baseline calibration algorithm, and performing only three consecutive bioreactor runs, enough user data can be gathered to construct a precise custom cell model. By using this overall approach and the final cell model, NIR spectroscopic monitoring can provide highly accurate glucose and lactate concentration sensing in real-time specific to the user cell line and culture medium.
For some purposes, simple monitoring of CPP such as glucose & lactate may provide significant value for production and quality control. However, it can also be coupled with intelligent bioreactor software systems to enable automated feeding of glucose for maintaining constant concentrations or dynamic control. More will be discussed about this in a later section.
In-line biomass measurement is an important PAT to understand the culture growth phase and cell viability in bioreactors in real-time. Sometimes referred to as bio-capacitance, electrical impedance sensors monitor cumulative charge polarisation across intact plasma membranes to estimate total live cell biomass. This technology has been widely adopted in biopharma as a popular method to calculate Viable Cell Density (VCD) in real-time. Like many users, we had encountered issues with adopting this technology, including reliability issues at higher cell densities and interference from some media components. However, we have innovated around these issues in several ways. Firstly, a thorough investigation of the cell physical size and growth dynamics (in suspension) during the cell culture timeline was completed to properly relate biomass data to proper cell counts. This is critical information that was collected by using a wellknown immortalised strain of CHO (Chinese hamster ovary) cells. Secondly, electrical impedance data was selected at multiple frequencies to overcome some types of interference from media components or other factors. And thirdly, we derived growthphase-specific data conversion algorithms that recognise transitions, and switch or compensate (as appropriate) in the cell culture timeline. For instance, we have found that calculation of VCD using bio-capacitance data from early and exponential growth phase culture can be used to derive an algorithm that provides high reliability specific to those earlier growth phases. However, this algorithm becomes a poor predictor of actual VCD in stationary or later stage cultures. This fact is likely due to changes in cell morphology that are reflected in later stage culture perhaps due to physiological shifts and changing protein expression profiles. It has been shown that in recombinant cell lines, the biologic expression of monoclonal antibodies is generally induced to higher levels when cultures achieve peak cell densities. In bioreactor culture, as CHO cells approach peak cell densities, glycolytic pathways transition to more oxidative phosphorylation metabolism, cell sizes may change and membrane composition also likely changes.
These events appear to result in a deviation from the calibration parameters applied for determination of VCD in early stages of culture. Hence, an entirely different data correlation model seems to be appropriate once cultures reach the peak VCD. By integrating and switching from early stage to late stage models at the appropriate time, the generation of accurate VCD data from bio-capacitance measurements can be achieved with appropriate CHO cell models. In our hands, using this approach, bio-capacitance can provide high accuracy in calculation of VCD data throughout the entire bioreactor run, including up to 100 x 106 cells per ml in cell culture.
As mentioned earlier, the employment of in-line PAT for real-time Process Control (PC) is an automation approach that has potential benefits for biotherapeutic drug manufacturing. A key example is to automate feed-control of nutrients in fed-batch culture. Spectroscopic technology for monitoring nutrient concentrations is limited in ways discussed earlier. However, it is universally agreed that glucose concentration is one CPP that has a direct impact on manufacturing performance. Hence, production cell lines are usually well characterised regarding glucose sensitivities and optimal concentration requirements for growth in bioreactor cell culture. Glucose concentrations have been shown to affect the metabolic state and growth rate of cells in culture, in addition to the expression yields, and post-translational modification of recombinantly produced biologic drugs.
To control glucose concentration in bioreactors in real-time with PAT, both accurate detection and feeding strategy are important for process control. PC considerations are obviously not unique to biopharmaceutical production, but rather have been widely studied and employed in many other industries such as chemical plants and oil refineries since the 1980’s. Early strategies, especially in the chemical industry, employed Proportional-Integral-Derivative (PID) control, largely dependent on feedback mechanisms. These PID-based methods work primarily via input sensing and rapidly reiterative feedback adjustment cycles. However, in bioprocess, in-line sensor data collection is slower and living systems take longer time periods to adjust and equilibrate to environmental or process changes. Hence the effectiveness of new prediction-based approaches can exceed the traditional reactive feedback manner. In bioprocess, we believe that PID-methods will increasingly be supplanted with more sophisticated feed-forward predictive control strategies requiring defined constraints factored into decision outcomes.
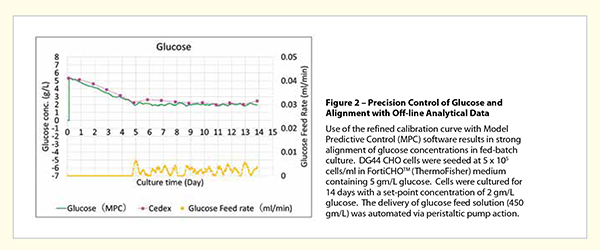
We have developed an intelligent CHO cell Model Predictive Control (MPC) algorithm for glucose-feed control that accommodates multiple constraints including VCD, growth phase, future-state, current concentration, feed-volume dilution factors, and selfcorrection based on differences in measured versus predicted data values. PAT-driven use of this dataadaptive MPC for automated delivery of glucose by peristaltic pump action, has been shown to provide precision control of glucose in fed-batch bioreactor culture, even in low glucose concentration ranges such as 1 gram/liter. With flexible programming features the dynamic regulation of glucose within a single bioreactor run can be implemented, enabling growth phase-specific concentration control, among other unique applications. The effects of this strategy for bioproduction have largely been unexplored mostly due to prior limitations in precision control of glucose concentrations during cell culture.

The integration and calibration of all these technologies and software requires expertise in cell culture, model building, spectroscopy, programming and data integration. Hence, it is less common for resourceconstrained organisations to explore sophisticated in-line bioreactor automation and process control technologies for biopharmaceutical production. Piecemeal assembly of the many components usually from many independent suppliers and software integrations are a challenging and time-consuming task.
In this article we have attempted to explain how we have addressed and solved many hurdles by creating a novel and fully-integrated bioreactor system that we call the BR1000, with unique software for operation and calibration. To maintain strong utility and precision in PAT sensor data but still allow customised use for CHO cell culture, the BR1000 bioreactor system contains programming with a significant degree of CHO cell application bias. Use of other cell lines or significantly divergent media compositions would likely not perform optimally due to these built-in biases. However, for applications with CHO-cell culture, the system we have assembled is likely the most accurate and efficient glucose control, lab-scale technology platform for recombinant monoclonal antibody process development. Currently we are working on next generation glucose sensing PAT control systems that provide similar process control benefits to CHO cell culture and automation for pilot and manufacturing scale bioproduction.
E. Tamburini, M.G. Marchetti and P. Pedrini. “Monitoring key parameters in bioprocess using near-infrared technology”. Sensors (2014), Vol. 14, p.18941-18959.
T. Yardley, D. Kell, et al. “On-Line,Real-Time Measurements ofCellular Biomass using DielectricSpectroscopy”. Biotechnology andGenetic Engineering Reviews, Vol. 17,2000, p.3-35.
B. Moore, A. Sanford and A. Zhang. “Case Study: The characterization and implementation of dielectric spectroscopy (biocapacitance) for process control in commercial GMP CHO manufacturing process”. Biotechnology Progress (2019), 35e2782. https://doi.org/10.1002/btpr.2782
Danny Chee Furng Wong, Kathy Tin Kam Wonq, Lin Tang Goh, Chew Kiat Heng, and Miranda Gek Sim Yap. “Impact of dynamic online fed-batch strategies on metabolism, productivity and N-glycosylation quality in CHO cell cultures”. Biotechnology and Bioengineering (2005), Vol. 89, No. 2, p.64-177.
Y.-S. Tsao, A.G. Cardosos, R.G. Condon, M.Voloch, P. Lio, J.C. Lagos, B.G. Kearns, Z. Lui.“Monitoring Chinese hamster ovary cells culture by
the analysis of glucose and lactate metabolism”. Journal of Biotechnology (2005), Vol. 118, Issue 3, p.316-327.
M. Buchsteiner, L-E Quek, P. Gray and L. K. Nielsen. “Improving culture performance and antibody production in CHO cell culture processes
by reducing the Warburg effect”. Biotechnology and Bioengineering (2018), Vol.115, Issue 9, p.2315-2327.
N. Templeton, J. Dean, P. Reddy and J.D. Young. “Peak antibody production is associated with increased oxidative metabolism in an industrially relevant fed-batch CHO cell culture”. Biotechnology and Bioengineering (2013), Vol.110, Issue 7, p.2013-2024.
T. Namatame and P. Cizdziel. “Making antibody medicines manufacturing a reality”. Pharmaceutical Manufacturing (2019), September Issue, p.30-36.