As the healthcare industry faces increased complexity and stricter requirements for drug development, market leaders are looking for new and innovative ways to address these concerns. As a result, pharmaceutical and biotech companies are looking for a strong partner that can meet these standards set by the industry and provide products that ensure the safest and most effective solution for their drug product. As two long-term industry players, Datwyler and SCHOTT have developed a new packaging solution, ideal for complexities faced during drug development: the Starter Pack.
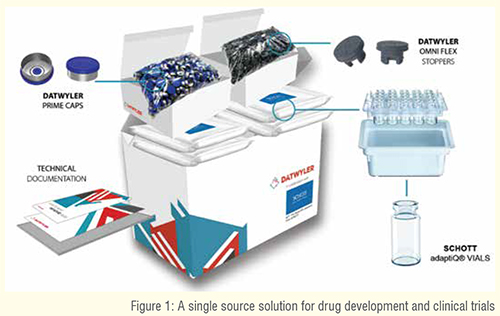
On average, the development of a new medicine takes 10 to 15 years and costs around US$2.6 billion. Drug development involves comprehensive clinical trial phases, of which less than 12 per cent of the candidate medicines make it into phase I, where clinical trials are eventually approved by the authorities. Driven by the latest developments in the field of medicine, such as cell & gene therapy, tissue engineering and regenerative medicine, the complexity is expected to increase even further in the future. Considering all critical aspects within drug development, Datwyler and SCHOTT developed the Starter Pack, which provides a range of compatible primary and secondary packaging components to be used in every drug development application – from drug discovery to drug delivery. The Starter Pack is designed to provide customers with a complete, robust, ready-to-use packaging system, easy to order and ready to be globally delivered in small quantities. The combination of the Datwyler Omni Flex stopper, Prime Cap, and SCHOTT adaptiQ® vial provides an ideal sealing solution which prevents leaks and other seal integrity concerns throughout manufacturing and handling.
Datwyler’s Starter Pack provides a range of compatible primary and secondary packaging components to be used in every drug development application. This allows manufacturers to incorporate highquality components and commercial packaging solutions into their testing and clinical trial strategy, including diagnostic research, clinical trials, and product launch. After that, the quantities can be ramped up.
The Starter Pack consists of Omni Flex stoppers, Prime Caps, and SCHOTT adaptiQ® vials. The products provided are ready-to-use (RTU) and have been sterilised in accordance with pharmaceutical and regulatory guidelines. All of Datwyler’s vial closure solutions are produced, controlled, and tested under the most stringent conditions in order to guarantee patient safety.
In addition to the Starter Pack, Datwyler offers its customers analytical and rubber compounding expertise during product selection and testing, which is based on the company’s extensive industry knowledge. With more than 100 years of multi-industry experience, Datwyler is aware of the challenges that customers are facing in their respective fields and can help them to find the right packaging solution for their needs. The implementation of these in-house programs presents customers with a comprehensive solution for parenteral packaging. The service offering includes pharmacopoeia and normative testing, functionality testing, and container closure integrity testing. Datwyler is a partner in drug development throughout the entire value chain, from the pre-clinical trial phase, over the phases 0 to III, up to the product launch.
The Starter Pack is designed to provide its customers with products that ensure complete Container Closure Integrity (CCI). The combination of the Datwyler Omni Flex stopper, Prime Cap, and SCHOTT adaptiQ® vial, provides an ideal sealing compatibility, preventing leaks and other seal integrity concerns throughout manufacturing and handling. Using standard CCI testing methods required by pharmaceutical and regulatory authorities, Datwyler can provide a total packaging solution for storing and administering sensitive drug products.
With the launch of the Starter Pack in collaboration with SCHOTT, Datwyler provides support for players in the healthcare industry as a strong partner throughout the drug development process. As the requirements and the complexity of medical solutions are rising and, therefore, posing multiple challenges for manufacturers in the field of drug development and drug testing, the Starter Pack is not only designed to provide the right packaging, but also to support the testing and launch process. With its commitment and trust in the innovation of healthcare companies, Datwyler is a reliable partner to improve patients' lives.