Gelatin’s legacy as a trusted excipient that complies with the highest international safety standards and regulations is well-established across the pharma industry, with the ingredient’s potential for biomedical applications also increasingly recognised worldwide. Its natural origin, cell-adhesive structure, high biocompatibility and biodegradability, as well as low immunogenicity, have made it an attractive material for biomedical solutions in a range of areas including regenerative medicine, drug screening and development, parenteral applications and hemostats.
Standard gelatins, however, do not comply with the purity requirements for most biomaterials. Pyrogens, including endotoxins, are common contaminants naturally found in native collagen, gelatin’s raw material, so extra purification steps need to be included in the production process to produce ultra-pure gelatins.
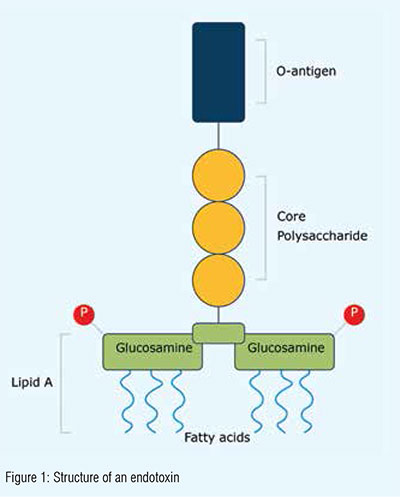
Endotoxins, which induce fever when released into the bloodstream, are a component of the exterior cell wall of Gram-negative bacteria. While they do not directly harm tissue, they can initiate mild to severe immune responses, as they act as an indicator for the presence of bacteria. Endotoxins can also trigger other cell types like stem cells and endothelial cells. In fact, any cell type containing the toll-like receptor-4 is sensitive to these molecules and as the potential to be affected in some way.
Depending on the concentration and exposure time, endotoxins can negatively impact cellular activity in terms of growth, morphology, differentiation, inflammation and protein expression even at very low levels (<100ng/ml). This means the biomedical application could fail or even endanger patient health.
To minimise these risks, biomedical manufacturers are increasingly turning to highly purified gelatin solutions. We have recently spoken to Tanja Vervust, Global Director Biomedical at Rousselot, to find out more about the current and future use of ultra-pure gelatins and collagens in the biomedical industry
In its native form, collagen is one of the most abundant structural proteins in all connective tissues in the human body. Treated with hydrolysis and purification procedures, it can be processed into ultra-pure gelatin and hydrolysed collagen (or hydrolyzed gelatin) solutions for markets such as regenerative medicine, parenteral formulations and hemostats.
Cost-effective and readily available ultra-pure gelatins and collagens are, for example, an ideal choice for tissue engineering as they can build tissue scaffolds to simulate a native tissue environment without activating the immune system.
In stem and endothelial cells specifically, using low-pyrogen gelatin as a biomaterial for scaffolds helps to minimise the risks of both immunogenicity and potentially tumorigenicity of the transplanted hydrogel. Ultra-pure gelatins can even act as a mechanical barrier for implanted organs, against a rejection of the immune system.
In addition, highly biocompatible, purified pharmaceutical gelatin helps provide drug protection, offers superior drug loading rates, and great flexibility in the control of drug release. It helps stabilise vaccines and other formulations and minimises side reactions while ensuring patient safety.
Thanks to gelatin’s foaming functionalities, ultra-pure gelatin can be designed to absorb more than 40 times its own weight and is widely accepted as an ideal material to control blood flow through hemostatic applications, mainly in the form of sponges, strips, powder or nanofibers.
Our portfolio of X-Pure gelatins and collagens offers manufacturers the purity needed to create effective biomedical applications that ensure patient safety.
Rousselot’s patented endotoxin removal process overcomes the limitations of other approaches that change the composition of gelatin or cause new impurities. Our customisable platform also allows to engineer any type of pharmaceutical and medical gelatin with different purity ranges at any scale, facilitating customisation to your needs while providing unparalleled consistency and purity. This makes X-Pure the most advanced range of ultra-pure gelatins and collagens (10EU/g) for biomedical applications across regenerative medicine, parenteral formulations and hemostats.
Our X-Pure range is deemed Generally Recognized as Safe (GRAS) by the US FDA and compliant with the US, EU, Japanese Pharmacopeias.
X-Pure can promote tissue regeneration in high-risk surgeries and cell-laden treatments in low-risk surgeries, while also supporting the healing process of deep uninfected wounds. In novel stem cell therapies, X-Pure increases cell growth and the viability of cell culturing and transport.
X-Pure’s potential in endotoxin-sensitive cell cultures was highlighted by a study carried out in collaboration with Ghent University1. A comparison on the viability of immune cells (THP-s) in a 10 per cent X-Pure gelatin solution versus standard hydrogels showed a significantly improved cell survival in X-Pure after 3 days of culture. This study highlights the potential of X-Pure as a valid and improved solution for endotoxin-sensitive cell cultures, as it also represents a solution to the batch to batch variation and the overload of growth factors associated with some other hydrogels.
Interestingly, gelatin hydrogels devoid from endotoxins can also be used for storage or transport of endotoxin-sensitive cells, as they significantly improve their survival. Preliminary results of a study carried out in collaboration with Ghent University showed that the addition of 10 per cent X-Pure gelatin to the medium significantly improved viability of endothelial cells after 3 days of storage at 4°C, even in absence of serum, and was significantly better compared to non-purified gelatin. It also aids the sustained or site-specific delivery of drugs, particularly in cancer therapy and orthopedic implants, and is an exceptionally safe gelatin solution for in-body hemostatic use.
High-quality and fully traceable, the X-Pure range is especially well-suited for applications that require frequent or highquantity administration in contact with cardiovascular or neural systems, as well as end-products that reside in the body for an extended period of time. Beyond this, Rousselot’s ultra-pure X-Pure gelatins and collagens can be used in combination with sensitive active ingredients like live cells, where impurities could impact the effectiveness, durability and/or viability of the application.
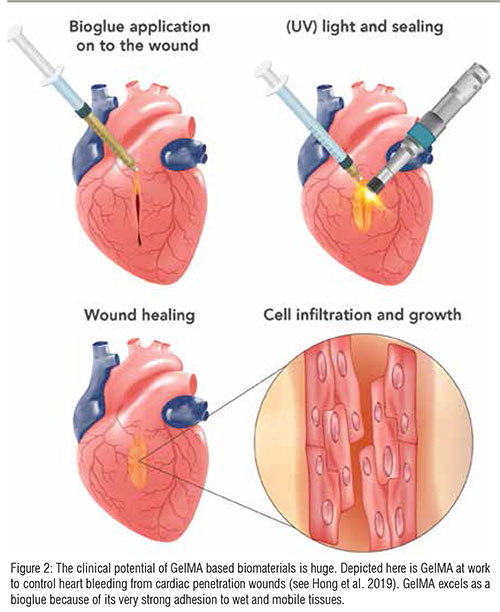
A polymerisable hydrogel material derived from gelatin, Gelatin Methacryloyl (GelMA) is an ultra-pure solution that is functionalised via photo-crosslinking and therefore holds great potential for the creation of tunable biological environments for culturing various eukaryotic cells at body temperature.
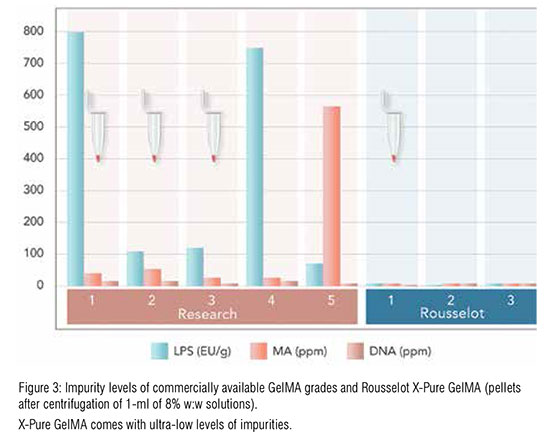
Over the past five years, gelatin methacryloyl (GelMA)-based biomaterials have increasingly moved into the spotlight and are widely used in various biomedical research applications, including bioadhesive, drug, gene and growth factor delivery, tissue engineering and biologyon- a-chip. Many of these concepts are now being translated into the clinic, making GelMA biomaterials an indispensable asset for medical innovation. Standard GelMA products, however, often carry high and variable levels of soluble impurities originating from either the raw material or the chemical synthesis process. The presence of these impurities such as endotoxins and MA residues are detrimental for in-body use and can also affect the success of in vitro applications.
To help biomedical manufacturers create high-quality, safe products, we have recently launched X-Pure GelMA, the first GMP-ready range of gelatin methacryloyl biomaterials suitable for preclinical and clinical applications, including 3D bioprinting, tissue engineering and regenerative medicine. Once gelatin has been obtained via hydrolysis, Rousselot’s unique purification process performs a two-stage routine, which results in GelMA solutions with ultra-low levels of pyrogens and residual methacrylic acid. This, combined with the full and validated traceability of Rousselot’s raw materials, guarantees an excellent safety profile, and consistent batch-to-batch quality and mechanical properties that ensure reliable results and shorter lead times to the clinic. The X-Pure GelMA production process is also scalable, meaning that variables can be changed to create solutions that are tailored to their specific purpose.
Tissue engineering is one of the most fascinating and trendsetting strategies for regenerative medicine. Its aim is to replace, repair or reconstruct injured tissue or organs with engineered functional tissue, biological implants and cell based multi-organ models attracting great attention for clinical, diagnostic and pharmaceutical research.
Within tissue engineering strategies, 3D bioprinting has been promoted as a state of the art tissue engineering technology, used to fabricate biomimetic scaffolds that are structurally and functionally relevant. 3D bioprinted scaffolds can support lineage-specific differentiation, intercellular communication between cells and their environment, and development into microstructures, such as capillaries, epithelia or organoids, meaning that it offers a great technological platform for reconstructing hierarchical tissue structures with full cellular functionality within the construct.
The 3D bioprinting process combines biomaterials with living cells to generate precisely controlled 3D cell models and tissue constructs. By optimally combining multiple cell types, growth factors and biomaterial compositions, highly complex and fully functional tissue constructs can be generated through self-organisation and maturation. In this process, the selection of a suitable biomaterial is of critical importance.
They should not only be biocompatible, but also have high water binding capacity and sufficient porosity to provide nutrient diffusion and cell migration. Additionally, mechanical properties such as stiffness and geometry have a great impact on cellular functions.
Gelatin’s versatility can represent a great advantage in bioprinting to obtain biocompatible cell laden constructs with high structural resolution and shape. However, to create bioprinted hydrogels with a good mechanical stability at physiological temperatures, gelatin needs to be crosslinked.
High gelatin purity is essential to secure proper cellular characteristics and functionalities in the engineered tissue that will ultimately be transplanted into the body to restore the damaged tissue / reduce the risk of tissue rejection.
Rousselot has been exploring the collagen molecule for over 125 years and knows its seemingly endless functionalities inside out. As the biomedical market evolves and new applications emerge, this space offers an exciting opportunity for companies with strong research and innovation capabilities like ours to further expand our expertise in the collagen market and help push the industry forward. This is why we have decided to do our part to advance biomedical science by delivering highquality, ultra-pure gelatin and collagen solutions that meet the demands of the biomedical market today and in years to come.
To find out more about Rousselot’s X-Pure range of ultra-pure gelatins and collagens for biomedical applications, visit www.rousselot.com/biomedical or contact: [email protected]