FPS is an Italian company specialised in the production of containment systems and micronisation equipment for Pharmaceutical, Biotech and Fine Chemical companies.
In the past 18 years FPS has grown to become the world leader for High Containment Isolators for fine powders with over 1,200 systems installed. FPS has a global technical team that supports these systems in the 40 countries where they are installed.
One of the reasons for this success is the high level of expertise of our staff. This expertise has been gained over 18 years of innovating in our field. The entire design and production process of the systems takes place in our state-of-the-art plant of Fiorenzuola d'Arda in Italy. This allows for optimum knowledge sharing & team work. As a result, each team player becomes an expert in his/her field and it really shows in the level of innovation and quality delivered.
Another reason for FPS success is our ability to listen to the specific needs of the customer, to focus on its process and to design a custom solution to address all those needs. It has been one of the strengths of FPS over the years.
Once the needs are well understood, the next step is to conduct the initial study of the project: a specialised team proposes a solution and works closely with the customer to ensure compliance with the technical specifications required.
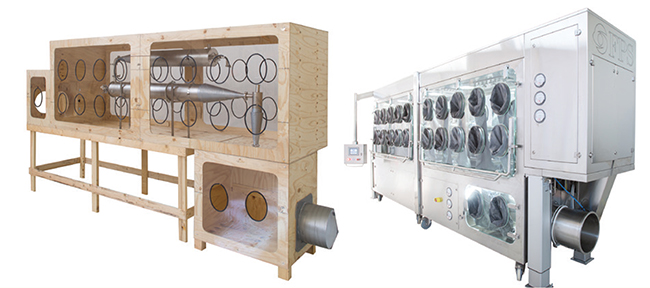
As far as containment systems are concerned, spray-drying is a commonly adopted technology in pharmaceutical to obtain a powder from a liquid solution. Given that pharmaceutical companies are handling High Potent API more than ever before, high containment solutions are necessary to safely operate the equipment.
In the past year, some of the isolators that FPS has designed and manufactured have been specifically to contain spray drying equipment. By using an isolator, pharmaceutical manufacturers want to ensure full operator protection without the need for bulky Personal Protection Equipment full-suits that can be cumbersome, costly and may provoke falls.

One of the key requirements that pharmaceutical companies have for isolator manufacturers is not to alter the current process. The goal is to avoid lengthy and costly process re-validation. Another requirement is to ensure excellent ergonomics to avoid operating errors, product loss and work injuries. The FPS design team is very familiar with those aspects and can also accommodate others. For example, one of FPS customers flagged that their multi-cultural team had a very wide variation in height. Their workers were very concerned that the isolator would be too low or too high for their own height and they worried about potential long-term health issues. Another customer had to fit the isolator into a very tight space and the equipment had to be brought in the installation room through narrow doors.
To address those issues FPS engineers worked in close collaboration with the end-user technical, safety and process engineering departments to design the isolator. Mock-up of those systems were built to simulate the activities and validate the technical solutions. Operators were also part of the process.
In the end these isolators had the following features:
- adjustable height for isolator optimum access to the processing equipment, with
- proper rating to work in hazardous conditions (according to the area classification)
- cooling of working chamber to keep the temperature constant (to counter heat created by the spray dryer)
- isolator creatively designed and delivered in small sections to pass narrow doors and assembled on site as one unit
"The users were very excited to do away with the cumbersome personal protective equipment. The ergonomics are much improved to perform the process and they like the flexibility of going in and out of the lab without gowning and disgowning" says Frederic Le Pape, the FPS America General Manager.
One customer uses a micronisation step in a separate chamber of the spray drying isolator and sees many benefits. For example, there is no need to use a split butterfly valve for product collection which are costly and time consuming to assemble, disassemble and clean.
Finally, the isolator protects the product from moisture during feeding and collecting phases for the spray dryer (and for the jet mill when used after the spray drying).
As always, the objective of FPS is to be of service to the Pharmaceutical and Fine Chemical industries. The mock-up is one of the tools that help us fulfill 100 per cent of the needs of our customers.