Microencapsulation possesses numerous advantages for many pharmaceutical applications: it's an effective means of converting a liquid to a solid, it provides taste masking, and it ensures controlled release capabilities. But partnering with a CDMO that possesses microencapsulation experience and expertise is integral to the product’s ultimate clinical and commercial success.
Today’s formulation challenges are as diverse as they are demanding–from increasingly insoluble new chemical entities (NCEs) to highly bitter drugs, to increased regulatory emphasis on patient acceptance–arriving at a final dosage form that has commercial potential is a more complex proposition than ever.
Microencapsulation, the process by which particles are co-formulated with a polymer or other excipients to improve palatability, modify release rate or enhance bioavailability, possesses a number of advantages for many pharmaceutical applications. An effective means of converting a liquid to a solid, taste masking a bitter active pharmaceutical ingredient (API), or ensuring controlled release, microencapsulation offers the encapsulated drug protection from environmental factors, flexibility in dosage form, and an array of other benefits.
There are several technologies available for microencapsulating drugs. Finding a contract development and manufacturing organisation (CDMO) possessing the experience and expertise necessary to optimise a drug’s efficacy and manufacturing during formulation is integral to the product’s ultimate clinical and commercial success. To do so requires companies to understand not only their own molecule, but the distinctions between technologies and among potential manufacturing partners, as well as the financial and therapeutic pitfalls often associated with unoptimised processes.
For oral drug formulations, there are three primary characteristics pharmaceutical companies must consider in their development paradigm to optimise a drug’s efficacy: solubility, permeability, and bioavailability. Arguably the single most important measure of a drug’s efficacy is its bioavailability, or the amount of unchanged drug which reaches systemic circulation. There are a number of physicochemical factors which impact bioavailability, including a drug’s solubility, its hydrophobicity, and its pKa. There are also various external factors, including whether a patient has consumed food, the relative health of a patient’s gastrointestinal tract, and whether a formulation has been tampered with prior to administration.
The reasons for poor bioavailability are equally diverse. They can include poor solubility, degradation in the gastrointestinal tract, interactions with food, insufficient time to ensure absorption, drug efflux pumps such as p-glycoprotein, hepatic first-pass metabolisation, and other factors. Addressing these requires a comprehensive approach to formulation, one that considers physical modifications to the size of the molecule, carriers that optimise dispersion, chemical modifications to the pH or salt content of the drug, and other methodologies.
There are several technologies that have been employed to enhance the solubility, dissolution, and bioavailability of drugs, as well as to improve their taste and overall palatability. These technologies include those which reduce the size of particles, an important first step in improving the control, stability, appearance, efficacy, and manufacturing of a drug. The most commonly-utilised technique to reduce particle size, micronisation, which can reduce API to a micrometer or, in some cases, nanometer size. This improves the solubility of poorly soluble APIs by increasing the particle surface area-to-volume ratio, accelerating the rate of dissolution. Considering the complexity of bioavailability, however, micronisation and other solubility-enhancing techniques can have a limited impact if the final dosage forms do not also exhibit uniform disintegration and performance. Simply stated, all facets of the product must be designed for consistent disintegration and dissolution.
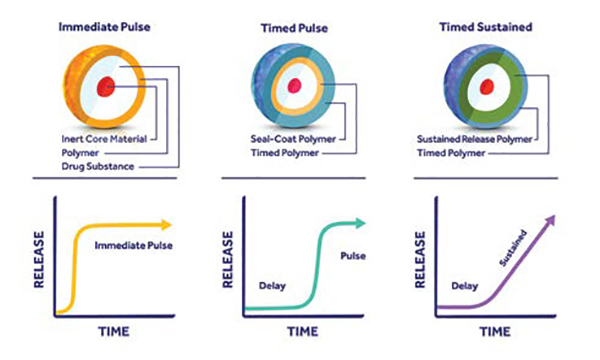
Adare, a global technology-driven specialty CDMO providing product development through commercial manufacturing expertise, focuses on oral dosage forms for the pharmaceutical, animal health, and OTC markets, and utilises propriety technology platforms for taste masking and customised drug release. Adare’s Optim m® technology is a melt-spray-congeal process that offers a wide range of modified release options in a powder format, allowing the such powder to mirror an extended-release tablet’s performance. Optim m-produced powder is compatible with many other dosage forms, including liquid suspensions, dispersible tablets, chewable tablets, and granular multi-dose devices.
The primary advantage Optim m offers when compared to other similar technologies is in the uniformity of the particles it produces. Adare’s Precision Particle Fabrication® technology enables particle uniformity with precision engineering, allowing for tighter control of release kinetics, and is the under-pinning technology for Optim m and Strat mTM (a long-acting injectable application). While there are a handful of melt-spray technologies on the market for microencapsulation, many of them are incapable of producing particles uniformly in size; even small variations in particle size can have implications for the final dosage form’s release rate and potency
Inconsistency in particle size can lead to content uniformity issues, which sometimes necessitates manufacturing modifications. For instance, during a coating process, if substrate particles are not uniform, a specific coating duration will result in different levels of coating for particles of varying sizes, with differing release rates or taste-masking, which can affect homogeneity of performance within a dose or from dose-to-dose. To address this problem, many manufacturers need to sieve their intermediate particles to create a more consistent particle size. This comes at a literal cost: as much as 30 per cent of what has been produced is subsequently discarded.

Adare’s Microcaps® technology is another example of a microencapsulation technology that can customise a drug’s release profile, provide taste masking, or combine otherwise incompatible APIs. With Microcaps technology, the raw API is directly coated with a thin layer of polymer. This does create a wider size distribution than that achievable with Optim m technology but enables a much higher drug loading on a weight basis. Moreover, Microcaps is an ideal choice for tastemasking high-dose applications, whereas Optimum has more flexibility in taste-masking and extendedrelease for moderate-to-low dose therapeutics. Like Optimum, drugs formulated using the Microcaps technology can be compounded into an array of dosage forms, including powders, dry syrups, orally disintegrating tablets, and Adare’s Parvulet dosage form, which mimics the texture of soft foods such as yogurt to increase swallowability for certain patient populations.
Microcaps and Optim m represent just two of Adare’s solutions for improving a drug’s efficacy and palatability. With a range of barriers and coatings, taste modifiers and suppressants, and API and API solubility modifiers, Adare’s portfolio of dosage form technologies is one of the most comprehensive in the world. These technologies, coupled with a long-standing focus on patient compliance, have positioned Adare as a leader in formulation for specific populations. Adare’s expertise also extends to pediatric formulations, which is a critical consideration for virtually all therapeutics.
By partnering with a CDMO like Adare, companies can identify a format that is the best fit for their drug and target patient population, thereby meeting increasingly stringent regulatory standards and, ultimately, improving patient outcomes through better patient adherence. If microencapsulation is the best choice for your drug, Adare has a portfolio of manufacturing approaches to get the job done.