The prevalence of biologic and biosimilar drugs is increasing in the pharmaceutical market. Due to the sensitivity of these drugs during storage, their complexity during administration, and specific packaging requirements, they demand high standards of packaging performance. These increasing demands mean that the requirements for drug packaging and elastomer components are evolving. As a leading industrialisation partner in the healthcare market, Datwyler is consistently striving to exceed these requirements by making improvements in the field of parenteral packaging solutions, specifically with regards to their Omni Flex fluoropolymer coating.

The primary goal in rubber formulation is drug product compatibility, especially in the case of highly sensitive drugs. This is achieved by reducing the amount of extractables and leachables for every new generation of rubber formulation for primary parenteral packaging components. In the 90’s, the disruptive breakthrough in this objective was the application of fluoropolymer coatings on top of rubber components. Datwyler’s Omni Flex was introduced in 1995 and has been the fastest growing product line for Datwyler ever since.
The uniqueness of Omni Flex begins with the way it is applied: it is the only spray-coated fluoropolymer coating that covers the total surface of the rubber component. Once the thin polymer layer is applied through a proprietary spray-coating technology, the products are further heat-treated to ensure a perfect adhesion to the rubber substrate and the coating itself turns into a pinhole-free, smooth, continuous fluoropolymer film. The Omni Flex coating provides a superior chemical inertness by acting as a barrier between the drug product and the rubber itself. Thus, the coating ensures the highest compatibility, which is especially important for sensitive drug formulations. This feature by itself explains the high success rate for Datwyler’s coated portfolio.
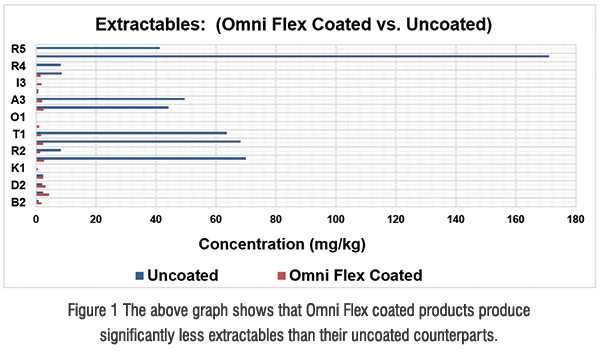
The scrutiny of any kind of particles in injectable drugs continues to grow and remains today the number one concern for many pharma companies and regulatory bodies. By using Omni Flex coated components, additional siliconisation during the final treatment, which is a known cause of subvisible particles, can be avoided. Traditionally, rubber closures are prone to stickiness and, therefore, silicone oil is added as a surface treatment during one of the washing steps. The silicone oil prevents clumping of bulkpackaged closures, helps with processing the components on filling lines, and, in the case of plungers, silicone oil lubrication helps to control break loose forces. Unfortunately, small silicone oil droplets may migrate from the stopper surface into the drug product after contact. This is even more likely when surfactants like polysorbate are used. Those hydrophobic oil droplets are of subvisible size and, by analytical techniques, typically counted as contribution to the total amount of subvisible particle load. The same is true for visible particles. Rubber particles, for example, are mainly generated by abrasion of the trim edge during handling. These particles are no longer an issue since the trim edge is completely covered by the Omni Flex coating.
A prerequisite to drug packaging success is the smooth functionality of a rubber closure, be it a vial stopper or a syringe plunger. In 2008, Datwyler became the first in the industry to launch an optimised design for the 1ml long and 1-3ml ISO-based plunger designs. Including a recessed trim edge and reducing the second and third rill in diameter and radius, the gliding behavior in prefilled syringes was further balanced without posing a risk to container closure integrity. The spray coating process offers design flexibility and any customised product design can potentially be coated, as long as it follows certain design rules determined by the Datwyler R&D Center of Excellence. Today, practically all standard available ISO-based component designs are available with the Omni Flex coating, ranging from 32mm infusion stoppers to 20mm and 13mm injection and lyophilisation stoppers, respectively. To complete the platform, 0.5ml, 1ml long, and 1-3ml plunger designs are also available. Finite Element Analysis (FEA) is applied to Omni Flex coated plungers to optimise the design and verify the response on gliding effect and container closure integrity. This model is a numerical method; the area to be investigated, e.g. a sealing component, is divided into a finite number of elements whose physical behavior has already been established. The behavior of the individual elements can then be efficiently calculated using appropriate algorithms. From the results, the behavior of the entire product is derived, and modification and optimisation solutions are proposed.
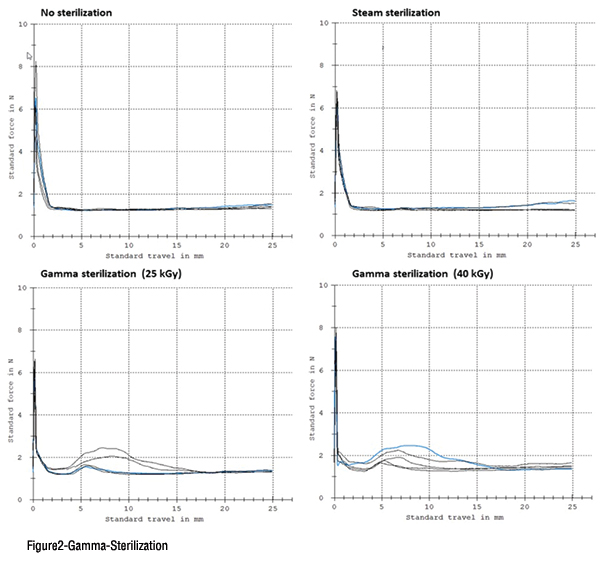
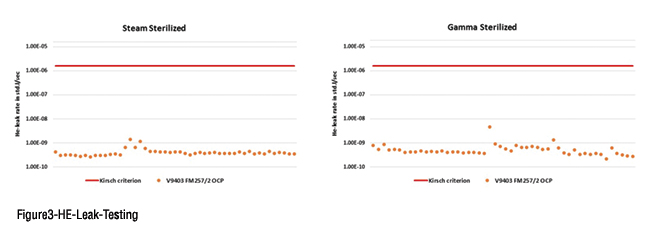
Omni Flex coated plungers are known for their consistency in glide force without any risk of shattering. In absolute value, the break loose and glide force is in a range similar to classic siliconised, uncoated plunger stoppers, for both steam and gamma irradiation sterilisation. Additionally, Omni Flex coated plungers can be processed by both insertion tube and vacuum placement, although the latter is the preferred method as it produces the least amount of deformation of the plunger itself.
In a world where self-administration and smart devices call for novel product designs and, hence, customised rubber closure designs, Omni Flex creates an opportunity to have such components in a fluoropolymer coated version. One important differentiator for Omni Flex is its resistance to gamma irradiation: both the rubber formulation itself, as well as, the fluoropolymer coating are composed in such a way that they can absorb and dissipate the irradiation energy without significant adverse effects on the properties. Therefore, Omni Flex is offered in both Ready-for-Sterilisation (RFS) and Ready-to-Use (RTU) configurations. In both instances, double laminated bags are used to properly pack the Omni Flex products and vacuum is applied between both bags. The latter serves as a visual indicator to check the bag integrity at the point of use.
Omni Flex, a proven technology for almost 25 years, has production lines available in all of Datwyler’s First Line plants in the US, Europe, and Asia, with all plants producing the same quality products worldwide. With all Omni Flex products produced under Datwyler’s highest-quality manufacturing standard, First Line, they can offer some of the lowest particle levels in the industry. Finally, local proximity is an advantage, as long storage and transport times can be avoided.
In conclusion, over its 25 years of existence, Omni Flex has become more than just a coated component. It has grown into a complete product platform, as an answer to the challenges posed by packaging highly sensitive drugs. With its Omni Flex technology, Datwyler plays a vital role in creating a safer environment and continues its mission to improve patients’ lives.