Quaternary ammonium compounds (Quats) such as Benzalkonium Chloride (BKC/BAK/BZK) are well-known antiseptics, effective against bacteria, fungi (yeast and mold) and algae.
But what about viruses? Quats are indeed effective against enveloped viruses. To name a few, activity against adenoviruses, enteroviruses, rotavirus, norovirus, influ-enza virus, severe acute respiratory syn-drome coronavirus (SARS-CoV), rhinovirus, chlamydia, HIV, herpes simplex and hepatitis A and B virus have been reported.
In these pandemic times, we are being reminded of the importance of using effective, yet safe antisep-tics. This has tremendous significance in the healthcare sector, where exposure to viral agents is high, and keeping workers and patients free from these agents is paramount.
Benzalkonium Chloride is a wellestablished antiseptic ingredient in several ophthalmic, nasal, oral and topical formulations. In topical products, it is often reported to be effective against viruses at 0.5% concentration or less.
Benzalkonium Chloride is recommended by several national agencies as an effective compound against coronaviruses, and it presents several advantages compared to using alcohol.
Quaternary ammonium compounds (Quats) such as Benzalkonium Chloride are well-known membrane-active agents interacting with the cytoplasmic membrane of bacteria and the plasma membrane of yeast.
The Quats’ hydrophobic activity also makes them effective against lipid-containing viruses. Quats disrupt lipid membranes, thus are more potent against lipophilic, enveloped viruses than against hydrophilic, nonenveloped viruses. Quats also interact with intracellular targets and bind to DNA.
Benzalkonium Chloride (BKC) is a well-established antiseptic ingredient in several ophthalmic, nasal, oral and topical formulations. It is effective against bacteria, yeast, molds and enveloped viruses. For example, activity against enteroviruses1, rota-virus2, norovirus3, 4, influenza virus5, severe acute respiratory syndrome coronavirus SARS-CoV6, rhinovirus7, chlamydia8, herpes simplex8 and hepatitis A virus9 have been reported.
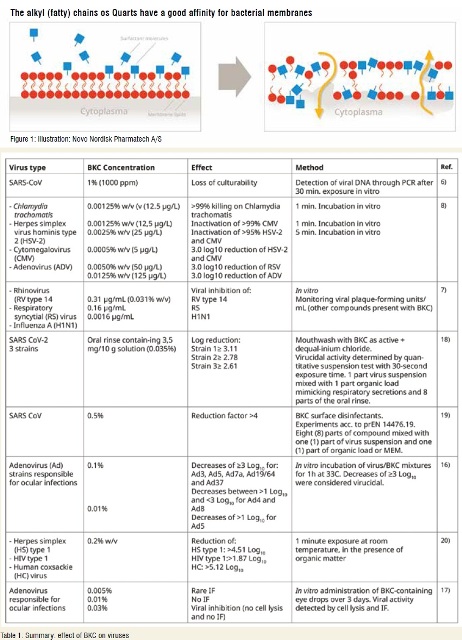
The cationic ‘headgroup’ of BKC is progressively adsorbed to the negatively charged phosphate heads of phospholipids in the lipid bilayer, and as a result, increases in concentration.
The consistent increase of BKC concentration results in reduced membrane fluidity and thus, the creation of hydrophilic gaps in the membrane. In addition, the alkyl chain ‘tail’ component of BKC further perturbs and disrupts the membrane bilayer by permeating the barrier and disrupting its physical and biochemical properties. Protein function is subsequently disturbed, and the combina-tion of these effects results in the solubilisation of the bilayer constituents into BKC/phospholipid micelles. BKC also interrupts inter-cellular targets and compromises the conformational behavior of DNA10.(Figure 1)
Fazlara and Ekhtelat11 report that through membrane destruction, BKC is efficient to act against bacteria, some enveloped viruses, fungi, yeasts and protozoa.
The length of the alkyl chain groups can also greatly affect the antimicrobial activity. Methyl group lengths of C12 to C16 usually show the greatest antimicrobial activity12.
Enveloped viruses such as HIV, hepatitis B virus and influenza virus are all susceptible to BKC113. BKC was found to inactivate influenza, measles, vaccinia, canine distemper, meningo-pneumonitis, rabies, fowl laryngotracheitis, Semliki Forest, feline pneu-monitis and herpes simplex viruses after 10 min of exposure at 30°C or at room temperature14. Saknimit et al.15 investigated virucidal activity of BKC against the canine coronavirus (CCV) and mouse hepatitis virus (MHV), Kilham rat virus (KRV) and canine parvovirus (CPV). BKC showed sufficient efficacy and could readily inactivate coronaviruses, whereas the two parvoviruses (non-enveloped) were relatively less susceptible.
A BKC concentration of 0.1% was found virucidal for Adenovirus Ad19, Ad3, Ad7a, Ad5 and Ad3716, which are strains causing ocular infections. Similar results were obtained at lower concentrations by other authors17.
Belec et al.18 demonstrated in vitro inhibition of adenovirus at varying concentrations of BAK with greater inhibition seen over longer exposure times.
Testing methods and concentrations differ greatly between scientific publications. We have summa-rized some results in Table 1.
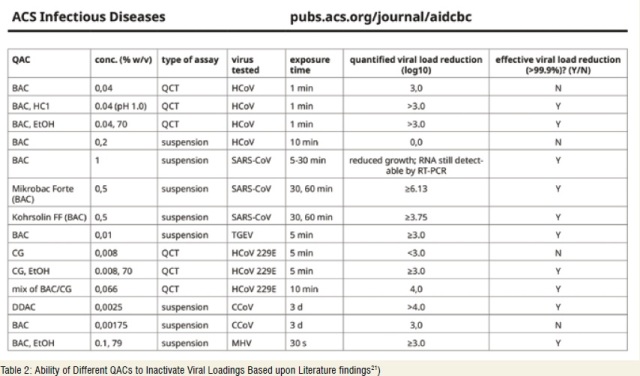
Numerous studies report a virucidal effect of BKC against coronaviruses, even if as pointed out by Schrank et al.21 in 2020, the cumulated data on BKC-based products against the family of known CoV’s is not uniformly asserted. This can be seen in their review of scientific papers: (Table 2).
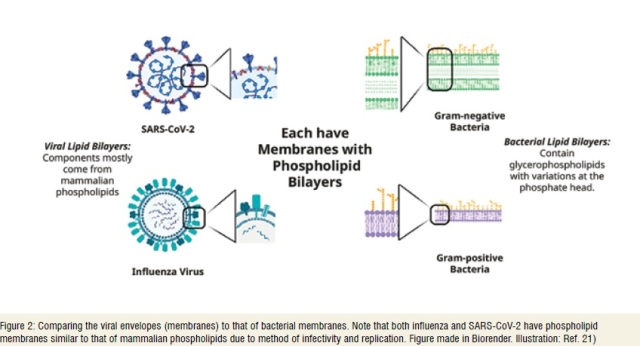
In general, Quats are reported to be effective against influenza viruses21. Hence, Schrank et al. pos-tulate the potential efficacy of such compounds against SARS-CoV-2, based on the comparable outer membranes structure (relatively similar phospholipid bilayers) between influenza and SARS-CoV-2 virus.(Figure 2)

The use of Quats-based disinfectants to deactivate SARS-CoV-2 has been recommended by several jurisdictions. For example, the US Environmental Protection Agency (EPA) has provided a list of suit-able disinfectant products: US Environmental Protection Agency ‘List N’: disinfectants for use against SARSCoV-2’ (US EPA, Washington, DC, 2020):
https://www.epa.gov/pesticide-registration/list-ndisinfectants-use-against-sars-cov-2
As of 16th April 2020, List N contained 370 recommended products. Of these, 171 (48%) products contain Quats ingredients alone, and a further 33 products contain Quats formulated with at least one other class of active ingredient.
Quats up to 0.2% are also among proven disinfectants suggested by the US CDC against enveloped SARS-CoV-2.
BKC remains on the FDA list for hand sanitizers: https://chemicalwatch.com/76526/us-fda-bans-28-substances-from-hand-sanitisers
Chemical-based disinfection is easily achieved by alcohol contact (ethanol or isopropanol) or other classic anti-viral and anti-bacterial compounds found in many commercial products. Alcohol offers momentary disinfection by contact and evaporation, while longer lasting effects that can be provided by less volatile active compounds such as Quats (BKC), remaining on surfaces. Alcohol-based hand sanitizers (ABHS) are less user-friendly on skin than Quatsbased, non-alcohol hand sanitizers (NAB-HS). ABHS predominate in healthcare settings given their low cost, however they are more worrisome due to their flammability and abuse potential.
For more information
If you would like to learn more about our pharmaceutical grade Quats portfolio and applications, please visit our website novonordiskpharmatech.com.
References:
1. WA Rutala, DI Weber, SL Barbee, MF Gergen, MD Sobsey. (2000) Evaluation of antibiotic resistant bac¬teria in home kitchens and bathrooms. Infect Control Hosp Epidemiol, 2000 21:132.
2. A. Bosch. Human enteric viruses in the water environment: a minireview. (1998) Int Microbiol 1998, 1:191 196.
3. S.L. Bolton, G. Kotwal, M.A. Harrison, E. Law, J.A. Harrison, Cannon JL. (2013) Sanitizer efficacy against murine no-rovirus, a surrogate for human norovirus, on stainless steel surfaces when using three application methods. Appl Envi-ron Microbiol 2013, 79:1368–1377. doi:10.1128/AEM.02843-12
4. A.M. Wilson, K.A. Reynolds, L. Jaykus, B. Escudero-Abarca, C.P. Gerba. (2020) Comparison of estimated norovirus infec-tion risk reductions for a single fomite contact scenario with residual and nonresidu-al hand sanitizers. American Journal of Infection Control, Volume 48, Issue 5, May 2020, Pages 538-544
5. J.J. Merianos. (2001). In Block SS (ed), Disinfection, sterilization, and preservation. Surfaceactive agents, 2001, p 63–320
6. Ansaldi F., Banfi F., Morelli P., Valle L., Durando P., Sticchi L., et al.. (2004) SARS CoV,
influenza A and syncitial respiratory virus resistance against common disinfectants and ultraviolet irradiation. J. Prev. Med. Hyg. 45, 5–8 7. Schmidbauer M. (2015). Dorithricin acts antiviral (in vitro). Pharm Ztg.160 (38):48-52.
8. Bélec L., Tevi-benissan C., Bianchi A., Cotigny S., Beumont-mauviel M., Si-mohamed A., et al.. (2000). In vitro inactiva-tion of Chlamydia trachomatis and of a panel of DNA (HSV-2, CMV, adenovirus, BK vi-rus) and RNA (RSV, enterovirus) vi-ruses by the spermicide benzalkonium chloride. J. Antimicrob. Chemoth¬er. 47 685–693.
9. J.N. Mbithi, S. Springthorpe, S.A. Sattar. (1990) Chemical disinfection of hepatitis A virus on environ-mental surfac-es. Appl Environ Microbiol 1990, 56: 3601–3604.
10. A.P. Golin, D. Choi, A. Ghahary. (2020) Hand sanitizers: A review of ingredients, mechanisms of ac-tion, modes of deliv-ery, and efficacy against coronaviruses. American Journal of Infection Control: Volume 48, Issue 9, P1062-1067
11. A. Fazlara, M. Ekhtelat. (2012) The disinfectant effects of benzalkonium chloride on some important foodborne patho-gens. American-Eurasian J. Agric. Environ. Sci., 12 (1), pp. 23-29
12. C.P. Gerba. (2015) Review: Quaternary Ammonium Biocides: Efficacy in Application Applied and Envi-ronmental Microbiology. Volume 81 Number 2, page 464 469
13. G. McDonnell, A.D. Russell. (1999) Antiseptics and disinfectants: activity, action, and resistance. Clin-ic. Microbiol. Rev., 12 (1) (1999), pp. 147-179
14. J.A. Armstrong, E.J. Froelich. (1964) Inactivation of viruses by benzalkonium chloride. Appl. Environ. Microbiol., 12 (2), pp. 132-137
15. M. Saknimit, I. Inatsuki, Y. Sugiyama, K. Yagami. (1988) Virucidal efficacy of physicochemical treat-ments against coro-naviruses and parvoviruses of laboratory animals. Jikken Dobutsu, 37, pp. 341-345
16. Romanowski E.G., Yates K.A., Shanks R.M., Kowalski R.P. (2019). Benzalkonium chloride demonstrates concentration-dependent antiviral activity against adenovirus in vitro.
J. Ocu. Pharmacol. Therap., 35 (5), pp. 311-314
17. Lazzaro D.R., Khaled Abulawi, Ph.D., and Mohammedyusuf E. Hajee, M.D. (2009). In Vitro Cytotoxic Effects of Ben-zalkonium Chloride on Adenovirus. Eye & Contact Lens 6: 329_332
18. Meister T.L. et al.. (2020). Virucidal Efficacy of Different Oral Rinses Against Severe Acute Respiratory Syndrome Corona-virus 2. J Infect Dis. 2020 Jul 29 : jiaa471
19. Rabenau H.F., Kampf G., Cinatl J., Doerr H.W. (2005). Efficacy of various disinfectants against SARS coronavirus. J Hosp Infect, 61, pp. 107-111
20. Wood A, Payne D. (1998). The action of three antiseptics/disinfectants against enveloped and non-en¬veloped viruses. Journal of Hospital Infection 38:283–95
21. Schrank C.L., Minbiole K.P., Wuest W.M. (2020) Are quaternary ammonium compounds, the work-horse disinfectants, ef-fective against severe acute respiratory syndrome-Coronavirus-2? ACS Infect. Dis, 6 (7), pp. 1553-1557