When it comes to efficiency and compliance in pharmaceutical water testing and cleaning validation, Sievers products and solutions offer unmatched productivity and peace of mind. Whether in the lab, at-line, or online, Sievers products can be used to simplify testing and lean out processes, all while complying with the latest data integrity guidelines.
SUEZ, through its Sievers product line of TOC Analyzers and solutions, is leading the way toward the future state of water testing.
Through its Sievers TOC Analyzers, SUEZ provides superior technology, design, quality, and service to the pharmaceutical industry. Whether in the QC lab, production, R&D, or engineering facilities, Sievers Analyzers can be deployed to meet compliance and lean out processes.
With a history of excellence in the pharmaceutical industry, Sievers product experts are available to help customers develop methods, determine feasibility, and implement new applications as needed. Sievers also provides Validation Support Packages for qualification (both instrument and oftware), Real-Time Testing (RTT), and cleaning validation. These packages help customers navigate the validation process and enable more efficient implementation.
Sievers instrument health and failure analysis reports help efficiently close out any TOC Out-of-Specification (OOS) investigations. With complete traceability of Sievers reference materials, vials, and analytical instruments, the services and corresponding documentation SUEZ provides to close out non-conformances are unmatched in the industry.
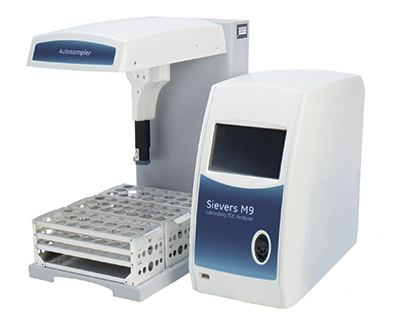
Beyond analytical instrumentation, Sievers certified services, consumables, and specialized support offer customers a complete TOC solution. From validation support and application expertise, to certified reference materials, failure analysis reports, and on-site services, SUEZ has the pharmaceutical industry covered to ensure customer success.
With customers' limited time and resources, it is not enough to simply manufacture instruments that don't provide added value. The industry demands not only tools that are accurate, but also those that exceed compliance requirements and will help increase productivity, automate processes, and troubleshoot effectively.
Starting with online monitoring and RTT of pharmaceutical waters, Sievers instruments enable better process understanding to perform corrective actions in real time and ensure quality. In alignment with industry guidance ASTM E2656, analytical instrument validation is required for real-time release testing. This can be achieved only with an analyzer, such as the Sievers 500 RL On-Line TOC Analyzer. The Sievers 500 delivers high quality online results, consistent with regulatory requirements for both process control and RTT. The robust Sievers 500 performance ensures the regulatory expectations can be met that are outlined in the FDA Process Validation Guidance document; ASTM E2656; and the ICH Q8, Q9, and Q10 international guidance. Also, WHO GMP Annex 2 Technical Report Series 970 encourages online TOC and conductivity monitoring.
When it comes to faster analysis, Turbo mode for Sievers M9 Analyzers enables real-time profiling of TOC for water testing, troubleshooting, or cleaning validation. Available for online, grab, or lab samples with an autosampler, Sievers Turbo mode provides four-second analysis time to quickly analyze samples or identify transient TOC excursions. This level of control and speed is vital to maximize productivity.
Sievers products are built to enable automation and efficiencywhether automating aspects of TOC analysis, enabling online deployment of TOC, or facilitating combined functionality such as simultaneous TOC and conductivity. For example, the Auto reagent feature on the M9 automatically establishes optimal flow rates for each sample, and the innovative Super iOS (Integrated On-Line Sampling System) for the Sievers 500 automates validation and system suitability testing. These features that automate aspects of TOC analysis make Sievers Analyzers easy to use and increase efficiency. Ultimately, this simplifies water testing and cleaning validation, allowing customers more time to focus on business growth opportunities.
When it comes to online deployment of TOC, leveraging the proprietary Sievers membrane conductometric technology enables the Process Analytical Technology (PAT) transition to be seamless from the lab to at-line or online deployment with like-for-like technology. Online monitoring maximizes efficiency and offers great opportunity for automation.
Sievers Lean Lab with the M9 streamlines compendia testing in the lab with simultaneous measurement of TOC and conductivity in a singe vial. By combining functionality, customers can save time, eliminate sample-handling issues, and minimize potential failures. Using the M9 for Stage 1 conductivity testing also enables automated instrument verification and sample data collection.
For compendia assurance of water quality, attributes such as TOC and conductivity can be tested simultaneously for USP <643> and <645> with Sievers M9 Analyzers. Known as Sievers Lean Lab, simultaneous testing decreases timeconsuming labor and data handling in the lab.
Conductivity testing with Sievers Analyzers enables automated, Stage 1 conductivity testing in an electronic, secured database that is fully compliant with 21 CFR Part 11 as well as the latest data integrity guidance. These added benefits of sample integrity, data integrity, and automation cannot be achieved with a bench top meter and probe.
In addition to compliance testing, both TOC and conductivity are well-suited analytical methods for cleaning validation. Using Sievers Analyzers, manufacturers can measure TOC and conductivity simultaneously and cover virtually all potential contaminants and compounds that may be present- whether organic or inorganic. This also provides even more powerful data analytics.
With Sievers products and validation support you can be equally assured about your software and data as you are about your validated Sievers TOC Analyzer. Sievers M9 Analyzers and DataPro2 software are fully compliant with 21 CFR Part 11 and the latest data integrity guidelines from the FDA and EMA. Sievers DataPro2 and DataGuard software offer complete solutions for electronic signatures and Audit Trails. To ensure system security, DataGuard restricts access through administratively controlled user accounts and automatically performs authority checks to verify that an individual can perform specific functions. It ensures that each system user has a unique user ID/password combination and offers the system administrator the ability to configure and enforce length and aging, login time-out, and different levels of access for user ID categories. DataGuard automatically generates an audit trail containing a date/time stamp for all actions performed and identifies the unique user name associated with each action. The audit trail is a separate, secure electronic record, safe from modification (intentional or unintentional).
With limited regulations but ample guidance, customers are re-examining their electronic records and data management systems to ensure compliance with regulatory and industry guidance. SUEZ is proud to provide solutions that are fully compliant with 21 CFR Part 11 and FDA data integrity guidance.
Company Details: SUEZ
6060 Spine Road, Boulder, Colorado 80301, USA
Telephone: +1 800 255 6964
www.sieversinstruments.com
A variety of resources related to Sievers products and pharmaceutical applications are available at www.sieversinstruments.com