Breast cancer resistance protein (BCRP)/ATP-binding cassette subfamily G member 2 (ABCG2) is an ATP-binding cassette (ABC) transporter that has been implicated as a molecular cause of multidrug resistance (MDR) in a variety of cancer cells.It got its name from being cloned from a multidrug-resistant breast cancer cell line, where it was discovered to impart resistance to chemotherapeutic drugs including mitoxantrone and topotecan. As a result, the FDA has designated BCRP as one of the main drug transporters involved in clinically relevant drug disposal.
1. INTRODUCTION
Many of the human ABC proteins are efflux transporters, and three of them, namely P-glycoprotein (P-gp, gene symbol ABCB1), the multidrug resistance protein 1 (MRP1, gene symbol ABCC1), and the breast cancer resistance protein (BCRP, gene symbol ABCG2), have been implicated to be the major efflux transporters responsible for multidrug resistance in cancer cells. Breast cancer resistance protein is a half transporter member of the ABCG subfamily (ABCG2) and was first identified by Doyle et al (1998) in a human breast cancer cell line selected for doxorubicin resistance in the presence of verapamil, an inhibitor of P-gp. Human BCRP is encoded by the ABCG2 gene which is located on chromosome 4q22. Like P-gp and MRP1, BCRP possesses a very broad substrate and inhibitor specificity that is different from, but substantially overlaps with that of P-gp or MRP1.
2. STRUCTURE AND TRANSPORT MECHANISMS
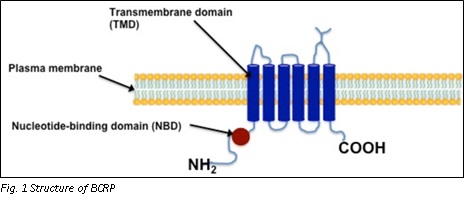
The human BCRP is made up of 655 amino acids and has a molecular weight of about 72 kDa. BCRP is expected to have six transmembrane helices and one nucleotide-binding domain near the N terminus. As a result, BCRP differs structurally from the other prominent ABC transporters, such as P-gp and MRP2, and is often referred to as a "half" ABC transporter. Three putative N-linked glycosylation sites are found in the protein. BCRP differs from most other ABC transporters in two ways, including P-gp and MRP1. First and foremost, BCRP is a half-ABC transporter with only one nucleotide-binding domain (NBD) and one membrane-spanning domain (MSD). [1]
P-gp and MRP1, on the other hand, have two tandemly repeated halves. Second, in BCRP, the NBD comes before the MSD, a domain organisation that is diametrically opposed to that of P-gp and MRP1. Because BCRP has such distinct structural features, it is possible that it operates in a very different transport mechanism than P-gp and MRP1. [2]
3. FUNCTIONS OF BCRP
BCRP is thought to play important physiological and pathophysiological roles in tissue and cellular protection, as well as in mediating physiological substrate homeostasis:
BCRP has been identified as an important component of organismal self-defence systems as an efflux transporter for xenobiotics and unwanted toxic compounds. BCRP is found on the luminal surface of micro vessel endothelium in brain microvasculature [3], and thus may be an important component of the blood-brain barrier.
The tissue distribution pattern of BCRP expression reflects its important role in protecting cells from potentially toxic xenobiotics and assisting with xenobiotic clearance from organisms.[4]
The protein acts as a xenobiotic transporter and may be involved in multi-drug resistance to chemotherapeutic agents such as mitoxantrone and camptothecin analogues. This protein has been found to be highly expressed in the placenta and to play a role in protecting the foetus from xenobiotics in the maternal circulation. [4]
BCRP is known to be a marker for pluripotent hematopoietic and tissue stem cells.
The transporter has been shown to play protective roles in blocking absorption at the apical membrane of the intestine, and at the blood-testis barrier [4], the blood–brain barrier, [4] and the membranes of hematopoietic progenitor and other stem cells.
At the apical membranes of the liver and kidney, it enhances excretion of xenobiotics. In the lactating mammary gland, it has a role on excreting vitamins such as riboflavin and biotin into milk. [4] In the kidney and gastrointestinal tract, it has a role in urate excretion. The protein also carries the Jr (a) antigen, which defines the junior blood group system. [5]
4. TISSUE EXPRESSION OF BCRP
BCRP expression is in the following organs:
4.1 BCRP expression in liver
BCRP is expressed on the canalicular membrane of hepatocytes in the human liver but not in the rat liver, indicating a significant species difference. [6] It is essential for the biliary excretion of drugs and endogenous compound conjugates. The BCRP transporter was discovered to be responsible for the biliary excretion of many drugs and their conjugates, including fluoroquinolone antibiotics, statins [7] and diabetes reagents. [7]BCRP was also found in the basolateral membrane of hepatocytes, with increased expression in chronic biliary diseases, implying adaptive mechanisms for reintroducing bile constituents into the systemic blood. The expression of BCRP in liver supportive cells may contribute to their resistance to cytotoxic agents.
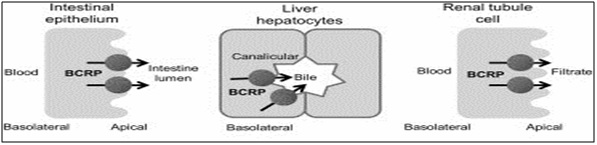
Fig.2 Expression of BCRP in Gastrointestinal Tract, in Liver and in Kidney
4.2 BCRP expression in kidney
Studies using immunohistochemistry and mRNA amplification show that BCRP is highly expressed in mouse kidneys and located on the apical membrane of cortical proximal tubules in the kidney. Regardless of the mouse kidney results, initial studies on BCRP expression in 50 human tissues found no ABCG2 mRNA in the heart, lung, skeletal muscle, kidney, pancreas, spleen, thymus, or peripheral blood leukocytes, and the transporter protein was not detectable by immunoblotting with two monoclonal antibodies. BCRP, on the other hand, was discovered in the proximal tubule brush border membrane of the human kidney by an independent laboratory using immunohistochemical methods. BCRP substrate efflux was found to be significant in primary human proximal tubule cells, suggesting that BCRP may play a role in renal drug excretion. [8]
4.3 BCRP expression in gastrointestinal tract
BCRP was discovered to be situated on the apical membrane of human intestine enterocytes. [9] The regional expression of BCRP was systematically investigated. BCRP was found to be most abundant in the duodenum, with expression decreasing from anterior to inferior in colonic divisions.
4.4 BCRP expression in blood brain barrier
Brain capillary endothelial cells attach firmly with one another to form the BBB, which differentiates the brain from the blood and provides protection to the CNS from distributing toxic compounds and damaging chemicals. A variety of carriers are expressed on the BBB, which either inhibit drugs and toxic compounds from reaching the brain or actively introduce nutrients and neurotransmitters into the brain. BCRP was discovered to be recognised in brain endothelium and to limit the CNS's exposure to its substrates. The lack of either P-gp or BCRP has no effect on the barrier function of the dual P-gp/BCRP substrates.The Cumulative inhibition of P-gp and BCRP has emerged as a palatable strategy for improving substrate delivery of drug to the CNS. [10]
5. Application of BCRP (Substrates and inhibitors)
The potential modulation of BCRP's physiological objectives by natural or synthetic compounds provides great opportunities for finding new anti-cancer drugs and valuable research tools used to research complex transporter ATP-binding cassettes. BCRP also has an important role to play in drug resistance for cancer treatment. Some inhibitors and substrates which play an important role are:
Inhibitors:
Fumitremorgin C
Fumitremorgin C (FTC, 5) is a prenylated indole alkaloid synthesized first from amino acids L-tryptophan and L-proline that has been identified from a variety of marine and terrestrial fungi [11]. FTC is the first BCRP inhibitor that is both potent and specific. FTC reverses resistance of topotecan, mitoxantrone, and doxorubicin in S1-M1-3.2 cells that expressed BCRP.
Tryprostatin A
Tryprostatin A (9) is a same molecule as FTC and is discovered from the marine fungus Aspergillus fumigatus, is being studied. Tryprostatin inhibits the G2/M phase of the cell cycle in tsFT210 cancer cells.[12]
Harmine
Harmine is a beta-carboline alkaloid with a wide distribution range that has been isolated from brown algae, various cyanobacteria, and sea animals. Harmine massively reduces resistance to anticancer medications like mitoxantrone and camptothecin, which are regulated through BCRP. Harmine's impact on MDR cells appears to be exclusive to BCRP, as this drug does not inhibit P-gp overexpressing cells [13]. Although Harmine was effective as a reversal MDR drug, its neurotoxicity and cytotoxicity hampered its clinical development, despite the fact that it is a promising lead for the development of BCRP reversal medicines.
Other examples of inhibitors are: 17 β -estradiol 17 β -estradiol 3-sulfate, dinitrophenyl-S-glutathione 4-methylumbelliferone glucuronide 6-hydroxy-5,7-dimethyl-2- methylamino-4-(3-pyridylmethyl) benzothiazole glucuronide 7-ethyl-10-hydroxycamptothecin.
Substrates:
BCRP has a high specificity, and many substrates interact to some extent with P-gp transporters (Robey et al., 2009). Despite the fact that BCRP was cloned from tumor cell lines, BCRP substrates are not restricted to chemotherapeutic drugs. Many drugs have been reported to be either substrates or inhibitors of BCRP, including tyrosine kinase inhibitors, antivirals, HMG-CoA reductase inhibitors, carcinogens, and flavonoids [14].
Examples of substrates include: 17 β -estradiol 3-sulfate 2.4-dinitrophenyl-S-glutathione 2-amino-1-methyl-6- phenylimidazo[4,5-b]pyridine 4-methylumbelliferone glucuronide 4-methylumbelliferone sulfate 6-hydroxy-5,7-dimethyl-2- methylamino-4-(3-pyridylmethyl) benzothiazole glucuronide 7-ethyl-10-hydroxycamptothecin.
REFERENCES
1. McDevitt CA, Collins RF, Conway M, Modok S, Storm J et al. (2006) Purifi cation and 3D structural analysis of oligomeric human multidrug transporter ABCG2. Structure 14:1623–1632.
2. Role of breast cancer resistance protein (BCRP/ABCG2) in cancer drug resistance. Natarajan K, Xie Y, Baer MR, Ross DD Biochem Pharmacol. 2012 Apr 15; 83(8):1084-103.
3. Localisation of breast cancer resistance protein in micro vessel endothelium of human brain. Cooray HC, Blackmore CG, Maskell L, Barrand MA Neuroreport. 2002 Nov 15; 13(16):2059-63.
4. Vlaming ML, Lagas JS, Schinkel AH (January 2009). "Physiological and pharmacological roles of ABCG2 (BCRP): recent findings in Abcg2 knockout mice". Adv. Drug Deliv. Rev. 61 (1): 14–25.
5. Kniffin CL (2013). "OMIM entry # 614490 – BLOOD GROUP, JUNIOR SYSTEM; JR". Online Mendelian Inheritance in Man.
6. Vander Borght S, Libbrecht L, Katoonizadeh A, van Pelt J, Cassiman D et al. (2006) Breast cancer resistance protein (BCRP/ABCG2) is expressed by progenitor cells/reactive ductules and hepatocytes and its expression pattern is influenced by disease etiology and species type: possible functional consequences. J HistochemCytochem 54:1051–1059.
7. Hirano M, Maeda K, Matsushima S, Nozaki Y, Kusuhara H et al. (2005) Involvement of BCRP (ABCG2) in the biliary excretion of pitavastatin. Mol Pharmacol 68: 800–807.
8. Huls M, Brown CD, Windass AS, Sayer R, van den Heuvel JJ et al. (2008) The breast cancer resistance protein transporter ABCG2 is expressed in the human kidney proximal tubule apical membrane. Kidney Int 73: 220–225.
9. Maliepaard M, Scheffer GL, Faneyte IF, van Gastelen MA, Pijnenborg AC et al. (2001) Subcellular localization and distribution of the breast cancer resistance protein transporter in normal human tissues. Cancer Res 61:3458–3464.
10. Agarwal S, Hartz AM, Elmquist WF and Bauer B (2011) Breast cancer resistance protein and P-glycoprotein in brain cancer: two gatekeepers team up. Curr Pharm Des 17:2793–2802.
11. Wang, Y.; Li, Z.L.; Bai, J.;Zhang, L.M.; Wu, X.; Zhang, L.; Pei, Y.H.; Jing, Y.K.; Hua, H.M. 2,5-diketopiperazines from the marine-derived fungus Aspergillus fumigatusYK-7, Chem. Biodivers. 2012, 9, 385–393.
12. Cui, C.B.; Kakeya, H.; Okada, G.; Onose, R.; Osada, H. Novel mammalian cell cycle inhibitors, tryprostatins A, B and other diketopiperazines produced by Aspergillus fumigatus. I. Taxonomy, fermentation, isolation and biological properties. J. Antibiot. 1996, 49, 527–533.
13. Ma, Y.; Wink, M. The beta-carboline alkaloid harmine inhibits BCRP and can reverse resistance to the anticancer drugs mitoxantrone and camptothecin in breast cancer cells. Phytother, Res 2010, 24, 146–149.
14. Robey RW, To KK, Polgar O, Dohse M, Fetsch P et al. (2009) ABCG2: a perspective. Adv Drug Deliv Rev 61 :3–13.