The process of producing pharmaceutical preparations from digital designs in a layer-by-layer fashion is termed as 3D printing. It has been acclaimed as a disruptive technology having the potential to make a paradigm shift in the conventional manufacturing of pharmaceuticals products which involves various unit operations like milling, mixing, granulation, drying and compression. It results in final products of different qualities. The quality of the product is influenced by loading of the drug, release of the drug, stability of the drug and stability of pharmaceutical dosage form.3D printed that created a novel era in pharmaceutical manufacturing was accepted by FDA. To overcome some of the challenges associated with conventional pharmaceutical unit operations, 3D printing is gaining more attention in the manufacture of pharmaceuticals in the future. 3D printing is capable of overcoming the difficulties relating to the drug delivery of peptides, potent drugs, water-soluble drugs, and the release of multi-drugs. On the other hand we can prepare patient specific or patient tailored medications on patient demand thus making safe administration of drug without any side effects or adverse drug effects. The major drawback to launch 3DP into the market is hindered by regulatory aspects.
3D printing (3DP) is a computer-aided design with unique technology of proto-typing layer-by-layer, fabricating 3D objects in the form of digital designs to achieve unmatched suppleness, time conservation and extraordinary manufacturing capability of pharmaceutical dosage forms. It works by depositing, binding and polymerisation through layering until the desired object is completed, thereby, applying a brilliant combination of chemistry, robotics and optics principles. 3D printing is also termed as Additive manufacturing (AM) or Solids of free-form (SFF). [1]
In personalized pharmacotherapy pharmacists can fabricate and dispense the personalized dosage instantly to the patients. Currently, the focus is to develop a patient-specific or tailored drug dosing system rather than using the conventional dosage forms, because an individualised dosage form will diminish all potential adverse effects. At present, dose modifications in solid drug delivery is achieved by dispensing multiple low dose tablets or by cutting up the larger sized tablets into smaller portions. Reportedly, over 3000 compounding pharmacies in the United States, dispensed more than 30 million prescriptions annually to provide customized drugs for specific patients.[2]
Although a number of advancements in drug delivery systems and formulations are being discovered, the oral route is still preferred, due to its simplicity and convenience. The advancement of 3D printing technology in the field of pharmaceuticals has brought about a drastic change in the manufacturing process and expected to be used as a large-scale industry in the future. The innovation of 3DP technology recently bequeathed very first 3D printed orodispersible tablet SPRITAM®(Levetiracetam) by Aprecia Pharmaceuticals and was approved by the US Food and Drug Administration (FDA) in 2015. This drug was indicated as an additional therapy for three prevalent types of seizures that were myoclonic and primary generalised tonicclonic and partial onset in adults and children with epilepsy.[3,4]
Developing personalized dosage forms has proven to be competitively cost-efficient in the long run. The complex geometries could be easily fabricated to develop multiple functional pharmaceutical products in a single tablet having diverse drug release kinetics. It provides a great possibility for personalization, e.g.drug eluting implants adjusted to a patient’s anatomical and physiological variations. Furthermore, maintaining the steady-state of drugs with a narrow therapeutic index requiring therapeutic drug monitoring e.g., Digoxin, Vancomycin, etc., in the terms of expediency, the rapid prototyping nature of AM is comparatively easy. 3D printing technology provides enhanced porosity to the tablets by layering both the active and inactive components, using aqueous fluid to bind the powder multi-layers together. This makes the pills easy to dissolve as it comes in contact with the liquid medium. It also becomes easy to swallow the higher doses of medicament in comparison to conventional tablets.
STEPS INVOLVED IN A 3D PRINTED DOSAGE FORM
ADVANTAGES AND LIMITATIONS OF 3DP IN PHARMACEUTICAL DRUG DELIVERY
In comparison to manufacturing of conventional pharmaceutical products, 3DP deals with a lot of interesting characteristics like:
a. Low-cost production.
b. Operating system is fast and therefore production rate is high.
c. Ability to customise products.
d. Rapid production of prototypes.
e. High drug-loading of potent or low dose drug with much-desired precision and accuracy can be achieved.
f. Reduces wastage of materials
g. Safety, efficacy, and accessibility of pharmaceuticals can be enhanced
h. Compliance to wide types of active pharmaceutical ingredients including poorly water-soluble, peptides and proteins, as well as drugs with a narrow therapeutic window.[6]
CURRENT 3D PRINTING METHODS
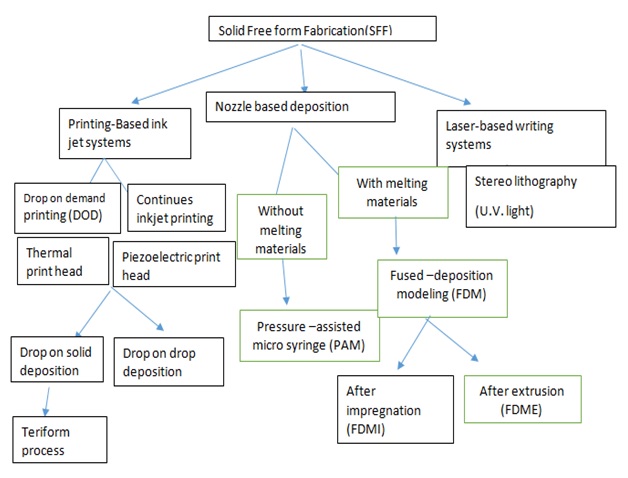
Fig. 1.3D Printing Technologies for Manufacture of Dosage Forms
1. INKJET PRINTING
This approach to personalize pharmaceuticals originates from the same technique of computer-operated inkjet printing. Pharmaceutically it was adapted by substituting ink with pharmaceutical solution that contains active ingredient and edible sheets known as substrates were used instead of normal paper. [7]
Dose alterations are done by altering the number of layers printed in a given area or changing the area to be printed. The choice of ratio of drug and excipients is such that it has the capability to print microdots on the substrate that is edible. The two main printing types employed under inkjet printing are Thermal Inkjet Printers and Piezoelectric Inkjet Printers. Printing-based inkjet systems is based on two techniques: Continuous Inkjet Printing (CIJ) and Drop-On-Demand (DOD) printing. Continuous inkjet printer involves uninterrupted passage of ink through an orifice of size range 50-80µm. The so passed liquid is then broken into droplets at specific speed and size at regular intervals of time. This process is done using a piezoelectric crystal and the parameters are controlled by establishing electrostatic field [8]. The broken droplets carry a charge and are distinguished by droplets of guard to reduce electrostatic repulsive forces between them. Hence the development of electrostatic field helps in transporting the charged droplets to the substrate.
The second technique i.e. Drop-on-demand technique is having numerous heads (100–1000) and uses thermal head or a piezoelectric crystal as a translator. The thermal head is used when the liquid is volatile in nature, and piezoelectric crystal is a translator of choice for several liquids. Besides, the thermal head reaches temperatures of up to 300 ºC, which implies that the use of solvents of high vapor pressure could cause the degradation of bioactive compounds. Hence because of this reason the pharmaceutical application of thermal print head is limited. Thepiezoelectric crystal shows a lot of variations rapidly which can lead to create a sudden change in volume. Droplets with the size range 10-50µmcan be produced by the heads. Piezoelectric printing technique is generally performed using less volatile and more bio compatible liquid at ambient temperature. Therefore, it is more prominently used in developing drug delivery devices.[9]
DOD technique is further divided into two subtypes:
1. Drop-On-Drop Deposition and, 2. Drop-On-Solid Deposition (powder bed fusion).
Inkjet drug printing is more prominent as it has an accurate control over combination of doses and drug release kinetics. The raw materials of inkjet printing should posses physical characteristics such as
1.Particle size should be smaller than 1 μmin order to avoid clogging of printer head
2.Viscosity needs to be < 20 cP and,
3. Surface tension should be between 30 and 70mN/m to promote smooth flow.[10]
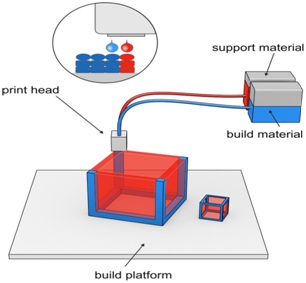
Fig. 2.Drop-On-Demand deposition technology
Formulating higher doses through this technology is difficult as it takes longer drying time for multiple layer printing in a particular area. Increasing surface area to sort this problem would in turn increase the size of the dosage form.
1.1. Selective laser sintering (SLS)
It is a technique, which is further derived from Drop-On-Solid Deposition Technique. Laser radiations are utilized in this technique to fuse the layered powder particles instead of using liquid ink. SLS is a typical procedure for 3Dprinting of metals. The final product is made up of sintered materials while the un-sintered materials share as the supporting structure and need post-printing processing to be removed. SLS has been well established in tissue engineering. It has not been employed in pharmaceutical manufacturing till now, probably due to a high energy laser beam causing possible decomposition of drug and other excipients [11].
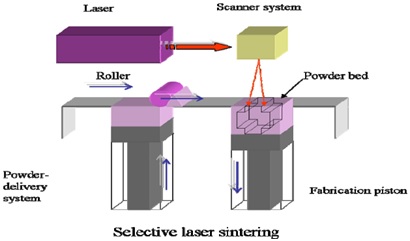
Fig.3.Selective laser sintering (SLS) technology
2. NOZZLE-BASED DEPOSITION SYSTEMS
In Nozzle-based deposition systems the active ingredients and excipients are blended thoroughly before 3D printing. The blend is then poured into the nozzle which comes out in a layer by layer fashion resulting in a 3 dimensional product. Depending on the type of material used the product is classified into Fused Deposition Modeling: In this the components are used in the melted form, and Pressure-Assisted Microsyringes: This method does not require melting of components.[12, 13]
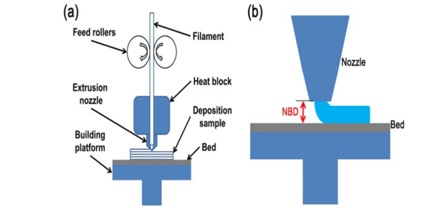
Fig.4. Nozzle based Extrusion System
2.1 Fused Deposition Modeling
In Fused Deposition Modeling a thermoplastic filament is extruded via a nozzle maintained at high temperature into a semi solid filament which is in fused state in a layer by layer manner. The computer software directs the printer head to extrude softened layers of thermoplastic filament in order to produce desired object. The process involves heating of material to just above its softening temperature followed by extrusion through a nozzle is then deposited in a layer by layer fashion, and solidified immediately. Therefore it is alternatively called as Filament Fabrication Drug loading. In this the formation of filament occurs through incubation in organic solvents [14].
This type of 3D printing relays on the thermoplastic polymers like polyvinyl alcohol (PVA), polylactic acid (PLA) or acrylonitrilebutadiene styrene (ABS). It is reported that acrylonitrile but adienestyrene is non-biodegradable polymer and not appropriate for3D printing of pharmaceuticals.[15]
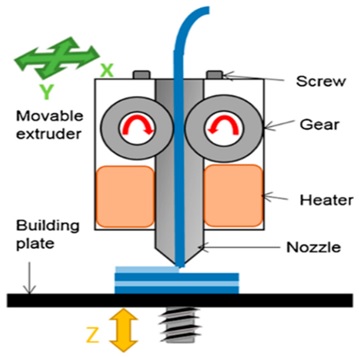
Fig. 5.Fused deposition modeling printing system
Advantages of Fluid Deposition modelling technique
Use of solvents could pose as health hazard and can degrade the active pharmaceutical ingredient as well. Use: Tissue printing substitutes or scaffolds of soft tissues.
3. LASER BASED WRITING SYSTEMS
3.1. Stereolithographic 3D Printing (SLA)
DISADVANTAGES
Pressure-Assisted Microsyringe technology
In this technique, a syringe extruder is used for depositing viscous and semi-liquid material, layer by layer, with the help of a pressurised air piston. Rheological parameters mainly viscosity and the apparent elastic limit are the keystones that govern the reproducibility of this method. Gels or pastes are formed by mixing suitable solvent(s) with optimum ratios of polymers to attain a viscosity suitable for printing.[17,18]
Advantage
Flows continuously and works at room temperature.
Disadvantage
Polymers through ultraviolet light or any other high energy light favors polymerisation reactions. Hence, it is also known as ‘Photopolymerisation’. So, for this technique, is limited to photosensitive or photopolymerisable materials primarily.
Stereolithography consists of a digital mirroring device, which induces a chemical reaction in the photopolymer resulting in the gelation of the particular exposed area. Unreacted functionalgroups attached to the solidified material in the first layer polymerises with the illuminated resin in the next adjacent layer, first ensuring adhesion and thereafter, the formation of layers. Certain backing structures are used to link the different parts of the object to avoid its collapse during the printing process. The final product is further treated to enhance the mechanical integrity and to polish or remove the attached supports to the printed subject by post printing process.[20, 21]
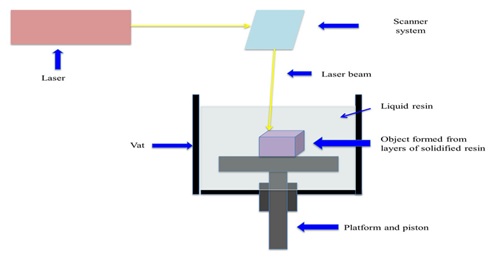
Fig. 6.Stereolithographic 3D printing (SLA) technology
CHALLENGES AND PERSPECTIVES
Advancement of technology in the pharmaceutical field is improving constantly and is providing possibilities to meet the needs of personalized drug therapy. Three dimension printing technique offers numerous advantages in fabricating drug delivery devices which are patient specific.3DP can successfully resolve the problems with the delivery of poorly water-soluble drugs, peptides, potent drugs, and the release of multi-drugs, etc. [22]
Application of 3DP is restricted in commercial market because of choice of binders and other excipients, and pharmaco-technical properties of finished product. Furthermore, these limitations can be overcome by improving the process performance and successfully combine 3D technique with novel drug delivery systems (NDDS) [23].
The main challenge to their exploitation for personalized pharmacologic therapy is likely to be related to the regulatory issues involved in the implementation of production models that may allow to efficiently change the therapeutic needs of individual patients into small batches of appropriate drug products to meet the quality requirements.[24]
Three-dimensional printing has become a useful and potential tool for the pharmaceutical sector. It focuses on patients’ needs for personalized medicine. In 3DP rapid prototyping can be done in no time, therefore increasing cost efficiency and manufacturing speed. In contrast to conventional dosage forms a barrier exists to establish the safety, efficacy, and stability of 3D printed dosage forms. The regulatory bodies are facing a tough challenge to put forth guidelines, laws, quality systems and safety rules for 3D printed pharmaceuticals. [25]
CONCLUSION
3D printing is gaining huge momentum in pharmaceutical industries despite the associated challenges. This advanced technology of 3D printing efficiently caters to the demand of patients for personalized medication. Certain unique systems, like personalized microneedle patch and drug-eluting implants, which were not possible with mass manufacturing, are now feasible by this novel technology. Apart from this, it also provides cost-effectiveness with high manufacturing speed, as rapid prototyping can be finished in minutes. It offers a great platform to bring dosage form manufacturing nearer to end-users with a more specifically adjusted dose to a patient in a safe and effective manner. Although 3D Printing technology is immensely promising for the fabrication of personalized drug delivery system, there are various technical and regulatory challenges for pharmaceutical applications which must be cleared for effective grounding of 3D printing technology. This technology opens the door to a new era of advanced drug delivery with built-in flexibility which is well suited for personalized/tailored medicine systems. It is anticipated that 3D printing would enable the development of mini dispenser and initiate the collaborative work between physician and pharmacist as for personalized therapy, the physician must understand the genetic sequence ofthe patient before starting any therapy followed by the pharmacist optimising the drug excipients ratio of decided drug concentration.
AKNOWLEDGEMENT
We would like to thank the Almighty for giving us the strength to study the topic and write the review. We would also thank our family members for their continuous support and encouragement.
CONFLICTS OF INTREST
There are no conflicts of interest.
FUNDING SOURCE
There is no source of funding.
REFERENCES
1.MusarratHussianwarsi,MohammadYusuf,sabakhan,MajedAl, Robaian,Mariakhan,HaseemAlsaab;
J.Current pharmaceutical design,24(2018)1-8.
2.A.Eswarkumar,G.Chinna Devi ,N.Sharada,kumar et al.,IJPSR,2019;Vol.10(4):1575-1581.
3.Diogo Jose Horst;J.Arc Org InorgChem Sci 1(12)-2018.AOICS.MS.1D.000109
4.WitoldJamroz,JonnaSzafraniec,MateuszKurek,RenataJachowicz;J.Pharm Res(2018)35;176
5.Preethy Ani Rose,PeterChristoper GV;J.AJPRD.2018;6(3):46-54.
6.Jassim-Jaboori A, Oyewumi M. 3D printing technology in pharmaceutical
drug delivery: prospects and challenges. J BiomolResTher 2015; 4: 1-3.
7.Fina F, Goyanes A, Gaisford S, Basit AW. Selective laser sintering(SLS) 3D printing of medicines. Int J Pharm 2017; 529(1-2): 285-93
8. Li Q, Guan X, Cui M, Zhu Z, Chen K, et al. (2018) Preparation and investigation of novel gastro-floating tablets with 3D extrusion-based printing Int J of Pharm 535: 325-332.
9. AsmatMajeed, Syed Naiem Raza and Nisar Ahmad Khan,Majeed et al. IJPSR, 2019; Vol. (3)10: 1025-1036.
10. Pamela Robles-Martinez , Xiaoyan Xu , Sarah J. Trenfield, AtheerAwad,Alvaro Goyanes , Richard Telford , Abdul W. Basit and Simon Gaisford. J.Pharmaceutics2019, 11, 274.
11.Fina F, Goyanes A, Gaisford S, Basit AW. Selective laser sintering(SLS) 3D printing of medicines. Int J Pharm. 2017;529(1–2):285
12.Andrew Kjar and Yu Huang.J.Pharmaceutics2019, 11, 390.
13.Bandyopadhyay A, Bose S, Das S. 3D printing of biomaterials.MRS Bull 2015; 40: 108-15.
14.Pere CPP, Economidou SN, Lall G, Ziraud C, BoatengJS,Alexander BD, et al. 3D printed microneedles for insulin skindelivery. Int J Pharm. 2018;544:425–32.
15. Zhang J, Feng X, Patil H, Tiwari RV, Repka MA. Coupling 3D printing with hot-melt extrusion to produce controlled-release tablets. Int J Pharm. 2017;519(1–2):186–97.
16. Goyanes A, Scarpa M, Kamlow M, Gaisford S, Basit AW, Orlu M. Patient acceptability of 3D printed medicines. Int J Pharm.2017;530(1–2):71–8.
17. Marks M, Alexander A, Matsumoto J, Matsumoto J, Morris J,Petersen R, et al. Creating thredimensional models of Alzheimer’s disease. 3D Print Med. 2017;3(13):1–11.
18. Gladman AS, Matsumoto EA, Nuzzo RG, Mahadevan L, Lewis JA. Biomimetic 4D printing. Nat Mat. 2016;15:413–8.
19.Ligon SC, Liska R, Stampfl J, Gurr M and Mülhaupt R. Polymers for 3D printing and customized additive manufacturing. ChemRev 2017; 117(15): 10212-10290
20.Skowyra J, Pietrzak K and Alhnan MA. Fabrication of extended-release patient-tailored prednisolone tablets via fused deposition modeling (FDM) 3D printing. European Journal of Pharmaceutical Sciences 2015; 68: 11-7.
21.Skowyra J, Pietrzak K and Alhnan MA. Fabrication of extended-release patient-tailored prednisolone tablets via fused deposition modeling (FDM) 3D printing. European Journal of Pharmaceutical Sciences 2015; 68: 11-7.
22.Yu DG, Yang XL, Huang WD, Liu J and Wang YG. Tablets with material gradients fabricated by three-dimensional printing. Journal of Pharmaceutical Sciences 2007; 96: 2446-2456.
23. Kumar AE, Devi GC and Sharada N. A review on novel approach to pharmaceutical drug delivery: 3D printing. Int J Pharm Sci & Res 2019; 10(4): 1575-81.
24. Katstra WE, Palazzolo RD, Rowe CW, Giritlioglu B, TeungP,Cima MJ. Oral dosage forms fabricated by three dimensionalprinting.J Control Release 2000; 66(1): 1-9.
25. Goyanes A, Robles Martinez P, Buanz A, Basit AW, Gaisford S.Effect of geometry on drug release from 3D printed tablets. Int J Pharm 2015; 494(2): 657-63.