Bioscintigraphy® also known as Gamma Scintigraphy is a non-invasive imaging technique used to visualize real-time in vivo performance and behavior of the drug or drug product. The integration of Bioscintigraphy with early formulation research and development studies will accelerate product development and lead to evidence based medicines.
Development of a new drug product remains a costly affair and figures of about $2.5 billion are quoted for development of one successful product. Surveys often show that more than 50% of the expenditure on drug product development is wasted because nine out of every ten drug products fail to reach market. More than 70% product development projects are terminated at early stages while failure rate of clinical studies are also high (~ 50%) with little-known reason. The huge cost implication has resulted in a drive for reinvention of the pharmaceutical industry and surge in development of ‘evidence based medicines (EBM)’ with the aim to reduce early as well as late stage attrition. One such EBM tool, which is well acknowledged by scientific community is “Bioscintigraphy®” also called as “Gamma scintigraphy”. It is the only available tool for direct assessment of critical performance parameters of the product and to establish correlation between drug kinetics and dynamics, which in vitro methods and clinical studies fail to achieve (Figure 1).
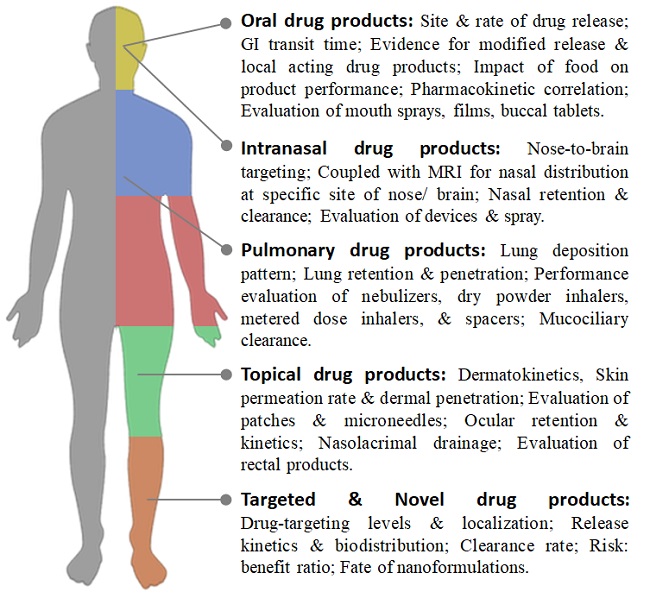
Figure 1. Bioscintigraphy in Drug Product Development
Novel drug products have been steadily developed to attain better therapeutic efficacy, enhance retention to treatment, and alleviate side effects. The complexity of these novel systems combined with the regulatory demands made the in vivo Bioscintigraphy almost compulsory. Bioscintigraphy studies can be performed in both preclinical and clinical setting and the data is acceptable by regulatory authorities as supporting evidence in New Drug Applications or Investigational New Drug Applications. With bioscintigraphy it is possible to visualize release, absorption, distribution, excretion, and dynamics of the drug products.
Bioscintigraphy Technique
Bioscintigraphy was initially introduced for diagnostic purpose, however, the prospects was quickly realized by the pharmaceutical industry and since then it has been adopted for evaluation and optimization of drug products. Bioscintigraphy procedure begins with the radiolabeling of drug or drug product achieved by incorporation of gamma emitting radionuclides. Technetium-99m (Tc-99m), due to its ready availability, short half-life (6.02 h), and ideal gamma energy (140 keV), is most widely used radionuclide. Tc-99m is obtained from radionuclide generators as sodium pertechnetate (Na99mTcO4), a non-reactive and soluble chemical form. The pertechnetate in the presence of suitable reducing agent such as stannous ion is reduced to a lower oxidation state Tc, which is reactive and forms chelate with drug or drug product. The amount of reducing agent, amount of radioactivity, pH, and contact time between radiolabel and drug, play important role in optimization of radiolabeling. The radiolabeling efficiency and stability is determined by instantaneous thin layer chromatography and thereafter in vitro studies are done to ascertain that radiolabeled drug behaves in a manner similar to drug. The radiation dose to which subjects are exposed during study is minimal and is often ten-times less than that received from a single X-ray. A gamma camera is used to detect the gamma rays emitted by radionuclide, which is representative of drug performance. Additionally, quantification is also possible by drawing region of interest. When imaging radiotracers are part of the drug product, they facilitate monitoring of its in vivo performance, which is not possible with a non-image guided approach.Data obtained from a well-designed Bioscintigraphy studies is accurate, comprehensive and clinically relevant, resulting in greater confidence in decision making, leading to more effective drug product development. Bioscintigraphy could be applied at all the stages of drug product development, to all types of drug products, administered by nearly all routes of administration, making it a versatile biological tool for drug and drug product evaluation.
Bioscintigraphy for Oral Drug Products
Despite the advent of novel routes for drug delivery, the majority of drug sare still administered as solid oral drug products such as tablets and capsules meant for rapid disintegration into the stomach. For these drug products, the rate and extent of absorption is dependent up on drug release, making in vitro dissolution as a surrogate for in vivo bioavailability. Regardless, about 70% of dissolution qualified products failed to show bioequivalence. Certainly, in vitro dissolution does not take into account all of the physiological factors that influence dosage form performance and the clinical bioequivalence only generate pharmacokinetic parameters. When pharmacokinetic profile differs from the one anticipated, presumptions are made to determine and resolve the cause. The requirement to understand the fate and behavior of the dosage form during its transit through GI tract has increased immensely not only to subside the developmental time and cost but also to provide evidence to regulatory bodies. Bioscintigraphy permits real-time visualization of the performance of the drug product and provide information about interaction between drug, dosage form and GI physiology, which is crucial for a successful product. Using bioscintigraphy, we can track GI transit of the drug product and gather information about drug release site and rate, residence time of dosage form at different anatomical sites and impact of food on drug product performance (Case study 1). For newer modified release drug products, Bioscintigraphy is even more useful, since it provides visual evidences for all the trepidations such as:(i)To which anatomical site does a modified release dosage form deliver? (ii) What is a drug release mechanism? (iii) For how long a gastro retentive dosage form resides in the stomach? (iv) Does a colon targeted dosage form delivers at the target site? (v) How rapidly does an enteric coated dosage form releases drug after gastric emptying?
Case study 1: Oral tablets for colon targeting were developed using different coatings. In bioavailability study it was observed that product Afailed, whereas product B passes. Bioscintigraphy was done to understand the problem with product A.
Study observations:
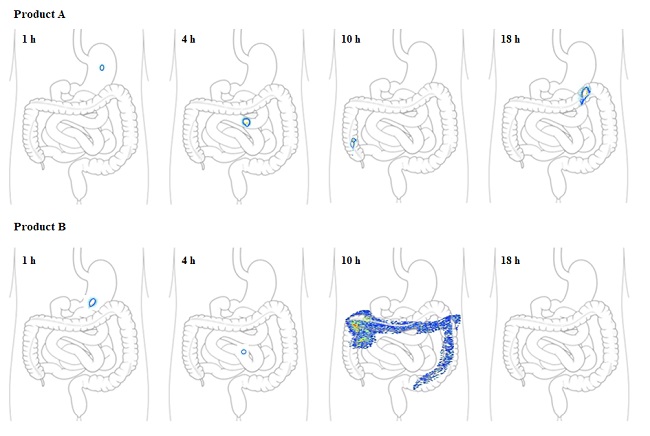
Inference: Product B could be seen intact in stomach and small intestine but releases drugas it reaches colon at 10 h. This indicates colon targeting property of product B. However, Product A could be seen intact in colonwith no drug release till 18 h. This might be due to failure of the coating to dissolve at colon pH leading to bioavailability failure.
Further, for locally acting oral drug products, bioequivalence studies are indicative of safety only and regulatory authorities suggest qualitatively assessments reflecting the presence of the active substance at the site of action. Bioscintigraphyis widely accepted as an incisive tool for evaluating the locality of in vivo release. Additionally, use of two different radionuclides allows for the concurrent evaluation of two processes and could be used to study the differential transit of the multi-particulate delivery systems or to study the effect of co-administered foods or liquids on GI transit of a drug product. The scintigraphic imaging coupled with pharmacokinetics could possibly be a gold standard for evaluating the fate of oral drug products.
Bioscintigraphy for Pulmonary Drug Products
The success of a pulmonary product involves a harmonic interaction between the drug formulation and the inhalational device and relied upon lung deposition pattern of drugs. Although, the prototype formulations are evaluated by particle size distribution (PSD) using cascade impactor (CI), the CI data have limited reliability owing to complex link between the PSD, the particle deposition in the lung, and the clinical response. To date, Bioscintigraphy is the most reliable method to generate claims of equivalence and to predict lung deposition (Case study 2). The technique involves addition of radiolabel to formulation followed by CI measurements to ensure that the deposition pattern of radiolabel represents drug molecule. Bioscintigraphy enables quantification of lung deposition pattern, anatomical site and extent of lung penetration, extrathoracic deposition and lung clearance of drugs. Bioscintigraphy has been extensively utilized for evaluation of in vivo efficiency of inhalational products and for performance assessment of inhalational devices such as nebulizers, dry powder inhalers, metered dose inhalers, and spacers. Bioscintigraphy also evaluates the influence of drugs on pharmacodynamics and lung functionality by studying effect on airway mucociliary clearance in healthy and diseased subjects.
Case study 2:Particles with different size and morphology were designed and their lung deposition efficiency was evaluated in healthy volunteers using Bioscintigraphy. The particles were delivered by dry powder inhaler.
Study observations:
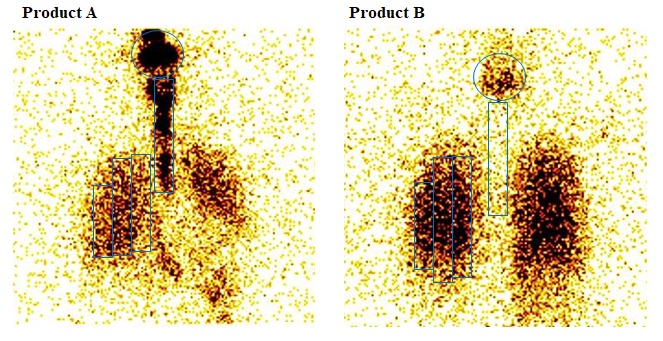
Inference: Product Ademonstrated high oropharyngeal deposition (63%) compared to product B (12%), whereas product B has 3-foldhigher and deeper lung deposition. By drawing ROI, deposition across lung can also be determined. Further, from images at different time-points, clearance rate can also be
Bioscintigraphy for Intranasal Drug Products
Intranasal (IN) delivery is emerging as a potential route for delivering small molecules, macromolecules, nanosystems and cellular therapies non-invasively to the systemic circulation, or local delivery to sinuses or targeting to brain via olfactory pathway. Preclinical scintigraphy is utilized by several researchers since it offer a great deal of evidence about the fate and site of the delivered molecule and enables optimization of IN therapies and their clinical translation. The clinical use of IN imaging studies have thus far been limited. However, combining SPECT scintigraphy (depicts 3D distribution of radionuclide)with MRI (depicts 3D image of anatomical regions)has perhaps the highest future prospects for generating proof-of-concept for IN delivery. The amalgamation of this dual-modality approach would quantify the precise amount of drug at the specific anatomical regions of the nose or the brain, providing efficacy data for IN formulations (Case study 3). Further, bioscintigraphy could also generate evidence to support statements to the regulating authorities that IN delivery results in no deposition to the lungs or other organs.
Case study 3: Coupling of bioscintigraphy and MRI to study IN delivery efficiency of IN liquid (A) and IN spray (B).
Study observations:
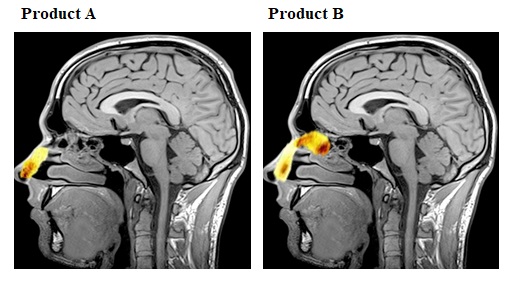
Inference: Both product A and B were distributed to all the nasal regions. The product B(16.9%) achieved higher deposition in the upper and middle posterior regions of the nose than product A (1.5%). No brain delivery and lung deposition was observed.
Bioscintigraphy for Topical Drug Products
Previous reports have mentioned that the bioscintigraphy has limited role in evaluation of topical drug products, however to our knowledge, a well-designed bioscintigraphy experiment can be applied to determine complete dermatokinetics of the topical drug product. Radiolabeling of topical drugs combined with imaging will predict contact time of formulation with skin, amount of drug left unabsorbed on skin, rate of drug permeated to systemic circulation, amount of drug penetrated to dermis and amount of drug in stratum corneum following detachment of stratum corneum by tape-stripping. Bioscintigraphy could be applied to understand influence of application site on drug permeation, to compare efficacy of dermal products and to evaluate performance of transdermal patches and micro needle arrays (Case study 4). On similar pattern, bioscintigraphy could be applied to determine ocular retention time, ocular permeation rate and nasolacrimal clearance of topical ocular products.
Case study 4: A microneedle patch has been designed for dermal application. Bioscintigraphy study on healthy volunteers (arm) was initiated to evaluate the dermal retention properties. Radiolabel microneedle was applied by applicator, the surface of the skin was cleaned and imaging was done thereafter.
Study observations:
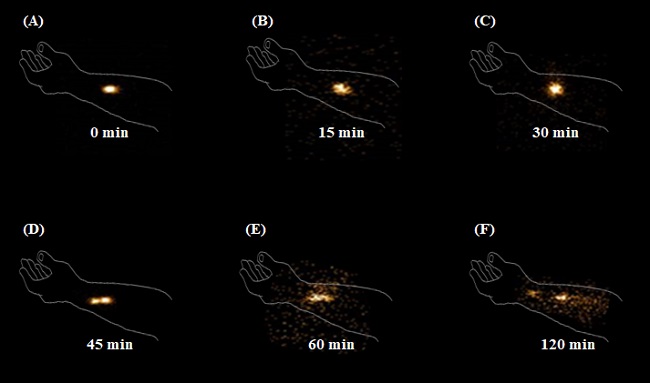
Inference: Image at 0 min indicates penetration of microneedles into the skin. Because of permeation into systemic circulation the radioactive count decreases with time. At 120 min, the displacement of radioactivity from site of application was observed indicative of slow skin diffusion due to blood
Bioscintigraphy for Novel and Targeted Drug Products
Bioscintigraphy plays a burgeoning role in the development and evaluation of novel drug products(TDP), particularly aimed for targeted therapy. An ideal TDP should be specific for the target cell and devoid of systemic effects. For these reasons, the optimization of TDP require Bioscintigraphy, with all its already‐known benefits. Image‐guided drug delivery allows determination of drug‐targeting levels and localization, release kinetics and bio distribution. The data also assess risk–benefit ratio by determining target: non-target drug distribution. SPECT and PET imaging also allows the study of the first‐pass drug elimination by liver and clearance by kidney. Additionally, radionuclides are conjugated with targeting ligands for diagnostic and the ranostic purposes. Ability of radiolabel to get attached to almost every molecule, allows Bioscintigraphy to assess in vivo fate and distribution of polymeric, lipidic and metallic nanoparticles.
Future Prospects
The advent of smart drug products with complex matrix and ingenious drug release kinetics, will call for a more decisive in vivo studies during the early development and regulatory phases and use of Bioscintigraphy with the purpose of obtaining effective, safe more reliable drug product will certainly be ever more. With adequate know how, this technique can be adopted for product branding strategy from marketing perspective. Further, the exploration of Bioscintigraphy for evaluation of herbals, nutraceuticals and food products should be task for the nearest future.
References
1. Singh AK, Bhardwaj N, Bhatnagar A. Pharmacoscintigraphy: An unexplored modality in India. Indian J Pharm Sci 2004;66:18-25.
2. Bhavna, Ahmad FJ, Mittal G, Jain GK et al. Nano-salbutamol dry powder inhalation: a new approach for treating broncho-constrictive conditions. Eur J Pharm Biopharm. 2009;71(2):282‐291.
3. Jain GK, Ahmed FJ. Adapalene pretreatment increases follicular penetration of clindamycin: in vitro and in vivo studies. Indian J Dermatol Venereol Leprol. 2007;73(5):326‐329.
4. Sharma N, SharmaA, Bhatnagar A, Nishad D, Karwasra R, Khanna K, Sharma D, Kumar N, Jain GK. Novel gum acacia based macroparticles for colon delivery of Mesalazine: Development and gammascintigraphy study. J Drug Delivery Sci Tech. 2019; 54. 101224.
5. Bioscintigraphy observations were taken from Research Projects conducted by the authors.