The era of nanomedicine-based products has revolutionised the science of drug delivery. Compared to the conventional delivery systems, nanotherapeutics possesses several advantages attributed to the nanometric dimension. The authors recommend the utilisation of nanotherapeutics in overcoming the regulatory hurdles via 505(b)(2) pathways in turn enhancing the clinical translation outcomes of nanotherapeutic formulations from bench to bedside.
Approval Pathways by FDA
Centre for Drug Evaluation and Research CDER, a fundamental component of Food and Drug Administration implements guidelines on various drug products, vaccines and biologicals [1]. Pharmaceutical industries seeking approval to market various therapeutic products must be in accordance with these guidelines. For a novel chemical entity which has not been used previously for a particular therapeutic indication, approval for the said entity could be granted via 505(b)(1) pathway provided its safety and efficacy has been well established. However, for molecules whose pharmacokinetic-pharmacodynamic profile has been testified by the innovator and has been already approved by the FDA through 505(b)(1) pathway could be established and marketed by other pharmaceutical companies via generic formulation development i.e. Abbreviated New Drug Application (ANDA), predominantly known as 505(j) pathway. 505(j) pathway requires the generic product to be therapeutically equivalent to the innovator product. The chief advantage of 505(j) pathway is the waiver of clinical trials owing to its status as a reference listed drug. In the midst of all these pathways, 505(b)(2), a hybrid pathway was presented by the FDA to accelerate the clinical translation of drug products [2]. This pathway could be applicable to NCE as well as the pre-existing drugs whose generic versions have been marketed. The versatility of this pathway lies in waiver of the clinical trials once the drug’s safety and efficacy has been established depending on the risk assessment category of the product. For products associated with low risk, i.e. slight difference with the innovator product, establishing bioequivalence is required whereas for products with medium and high risk, bioequivalence studies are not required. It has also been proven to be a safe strategy in evading a known risk compared to other pathways [3]. Additionally, the remarkability of this pathway in hastening the research and development lies in proving the drug product of the manufacturer to be superior compared with the existing products of the innovator.
Avenues in 505(b)(2) Pathway
Various avenues in improving the therapeutic efficacy of the drug product includes use of different doses forms, different routes of administration, solubility and permeability enhancements leading to bioavailability enhancement, enhancing targeting efficiency and diminishing the adverse effects, minimising the food effect, repurposing of drug moieties, etc. [4,5]. Figure 1 depicts various avenues for 505(b)(2) pathway which could be potentially used to overcome the regulatory hurdles proposed by the other pathways.
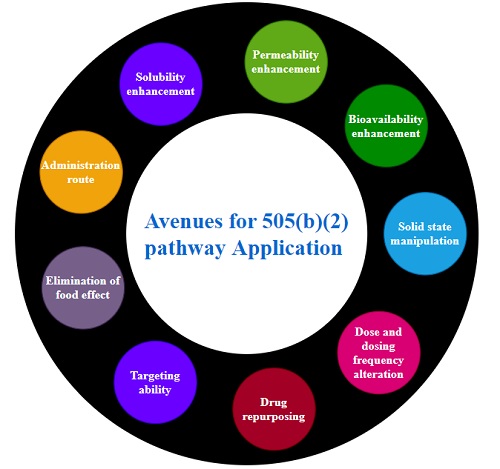
Figure 1 Avenues for 505(b)(2) pathway application
Role of Nanotherapeutics in Traversing the Regulatory Hurdles
Nanotherapeutic strategies could be used as a potential ally in achieving superiority of the drug product. FDA drafted guidelines for industry on drug products consisting of nanomaterials. According to US FDA, the term ‘nanomaterial’ is defined as any external or internal surface ranging within the nanometric dimension (up to 1000 nm) which alters its physicochemical properties to a significant extent [6]. In the last few decades, carrier linked as well as carrier free nanomedicine has emerged as a powerful tool in drug delivery. From the advent of Doxil® approved by US FDA in 1995, the clinical translation of nanomaterial based products which was once called a myth became a reality. Since then, a number of nanomaterial based products are dominating the market with their superior therapeutic efficacy compared to conventional products [7]. In context with 505(b)(2) pathway, the supreme advantage of developing nanotherapeutic products is the superiority imparted by the nanometric dimension compared to the conventional route. The nanometric dimension enhances solubility and permeability which in turn improves bioavailability thereby diminishing dose and dosing frequency [8]. Additionally, formulations containing lipid/polymer as a carrier could help in controlling and sustaining the drug release, improving bioavailability through lymphatic uptake, reduce the food effect on drug absorption, etc. Superiority designs could be utilised to qualitatively and quantitatively prove the enhancement in product properties compared to the innovator [9]. Statistical data assessment through the tools like student’s t test, ANOVA, etc. could quantitatively determine the extent of superiority via ‘p value’ based on the confidence interval [10]. Market entry of nanotherapeutic drug products is possible with the help of 505(b)(2) pathway by circumventing the innovator’s conventional product patentability and exclusivity [11]. Hence, the focus on 505(b)(2) pathway is of tremendous importance as the manufacturers need not wait until the end of patent term and do not require to be bioequivalent with the innovator [12]. Rather the product must be superior compared to the innovator to gain approval through 505(b)(2) pathway. This pathway helps in overcoming the innovator monopoly, reduce the pricing of medications, promotes research and development of improved products and hastens the clinical translation of various products inclusive of Nanomedicines [13].
Conclusion
Nanotherapeutics possess several attributes compared to the conventional delivery systems. These attributes could be used to overcome various regulatory hurdles, innovator monopoly and exclusivity via the 505(b)(2) pathway. This article emphasises on the significance of 505(b)(2) pathway in hastening the clinical translation of products and additionally the employment of nanotherapeutics in overcoming the regulatory hurdles which would in turn facilitate faster entry of nanomedicine based products in to the market through quicker journey from bench to bedside.
Conflict of interest
The authors declare that there is no conflict of interest.
References
[1] U.S. Department of Health and Human Services Food and Drug Administration, Guidance for Industry on Drug Products, Including Biological Products, that Contain Nanomaterials - Guidance for Indutry, 2017.
[2] W.F. Salminen, M.E. Wiles, R.E. Stevens, Streamlining nonclinical drug development using the FDA 505(b)(2) new drug application regulatory pathway, Drug Discov. Today. 24 (2019) 46–56. https://doi.org/10.1016/j.drudis.2018.07.005.
[3] V. Prasad, M. Biswas, S. Vishal, Is 505(b)(2) filing a safer strategy: Avoiding a known risk?, Int. J. Intellect. Prop. Manag. 7 (2014) 1–14. https://doi.org/10.1504/IJIPM.2014.062781.
[4] I. Freije, S. Lamouche, M. Tanguay, Review of Drugs Approved via the 505(b)(2) Pathway: Uncovering Drug Development Trends and Regulatory Requirements, Ther. Innov. Regul. Sci. (2019). https://doi.org/10.1177/2168479018811889.
[5] S. Shah, S. Nene, N. Rangaraj, R.S. Raghuvanshi, S.B. Singh, S. Srivastava, Bridging the gap: academia, industry and FDA convergence for nanomaterials, Drug Dev. Ind. Pharm. 46 (2020) 1735–1746. https://doi.org/10.1080/03639045.2020.1821055.
[6] J. Jampilek, J. Kos, K. Kralova, Potential of nanomaterial applications in dietary supplements and foods for special medical purposes, Nanomaterials. 9 (2019). https://doi.org/10.3390/nano9020296.
[7] F. F., G. A., G. O., R. A., K. M., H. M.R., Nanopharmaceuticals and nanomedicines currently on the market: Challenges and opportunities, Nanomedicine. 14 (2019) 93–126. http://www.embase.com/search/results?subaction=viewrecord&from=export&id=L625758504%0Ahttp://dx.doi.org/10.2217/nnm-2018-0120.
[8] N. Rangaraj, S.R. Pailla, S. Shah, S. Prajapati, S. Sampathi, QbD aided development of ibrutinib-loaded nanostructured lipid carriers aimed for lymphatic targeting: evaluation using chylomicron flow blocking approach, Drug Deliv. Transl. Res. 10 (2020) 1476–1494. https://doi.org/10.1007/s13346-020-00803-7.
[9] A.M. Brintrup, J. Ramsden, A. Tiwari, An interactive genetic algorithm-based framework for handling qualitative criteria in design optimization, Comput. Ind. 58 (2007) 279–291. https://doi.org/10.1016/j.compind.2006.06.004.
[10] P.R. Freeman, L. V. Hedges, I. Olkin, Statistical Methods for Meta-Analysis., 1986. https://doi.org/10.2307/2531069.
[11] S. Dahiya, R. Dahiya, New Deliveries and Nanomedicines: Commercial Aspects and Business Perspectives, in: Nano Med. Nano Saf., 2020: pp. 579–609. https://doi.org/10.1007/978-981-15-6255-6_22.
[12] K. Phelps, Using 505(b)(2) to Solve Shortfall from Generic Cliff, Appl. Clin. Trials. 24 (2015) 22–28.
[13] P. Rathee, S. Tripathy, S. Khatter, B. Patra, P. Murthy, H. Dureja, 505(b) (2) - A smart pathway to differentiate from competitive, low margin environment of generics, J. Generic Med. Bus. J. Generic Med. Sect. (2021) 174113432098786. https://doi.org/10.1177/1741134320987866.