The journey of plant miRNA from our food sources to our elementary canal and then to various organs where they can regulate several physiological processes has received very little attention. The present review describes the biogenesis, transport, and probable functions of plant miRNAs. It also discusses the ability of plant miRNA to direct effector molecules and establish molecular signalling in order to control a variety of human diseases. Plant miRNAs have the potential to regulate gene expression and hence physiological functions across the kingdoms. This study also explains the linkage of plant miRNA to modern diseases such as cancer, diabetes, obesity, heart and vascular diseases, and corona virus disease
Introduction
Natural compounds are becoming more popular as reliable sources of disease prevention due to their lower toxicity, ease of availability, and minor complications in therapeutic interventions. "Let food be thy medicine, and medicine be thy food," Hippocrates said nearly 2500 years ago, implying that both food consumption and disease occurrence influence an individual's health (Aman and Masood, 2020). Bioactive compounds such as vitamins, phytochemicals, polyphenols, flavonoids, flavonols, and carotenoids have been shown in epidemiological studies to have beneficial effects on human health and may lower the risk of a variety of diseases ranging from cancer to viral infections (Seca and Pinto, 2018). The Nobel Prize in Physiology or Medicine was awarded to Fire and Melo in 2006 "for their discovery of RNA interference gene silencing by double-stranded RNA." As a result of this ground-breaking research, many early articles on RNAi focused on using small-interfering RNAs (siRNAs) to silence genes of interest. However, a new class of RNAi molecules has recently emerged, with miRNAs playing a more prominent role in research and technology (de Almeida et al., 2018). The most important genomic regulators are microRNAs (miRNAs), which control 1–4 per cent of all human genes. Small non-coding RNAs (miRNAs) are defined as having 21–25 nucleotides. In gene expression and post-transcriptional regulation, the 3'untranslational region (3'UTR) binds for mRNA degradation and translational suppression (Chaiwangyen, 2021). As previously revised, miRNAs modulate mRNA content at the post-transcriptional stage to aid in many important biological activities such as embryonic development, cell maintenance, chemical signalling, and apoptosis. Almost 2000 miRNAs in humans have now been identified using several in-silico methods. These anticipated miRNAs regulate a wide range of cellular and physiological mechanisms (Kura et al., 2019). A change in miRNA (microRNA) expression has been linked to the onset of several diseases, including cancer. As a result, miRNA has emerged as a potential marker for assessing and diagnosing disease progression (Kang, 2019).
The process of miRNA biosynthesis
miRNA biogenesis is classified as either canonical or non-canonical. The canonical route in miRNA transcriptional regulation consists of four sequences of events. The miRNA gene is first converted to pri-miRNA, then the microprocessor complex converts pri-miRNA to precursor miRNA (pre-miRNA), the exportin 5 (EXP5/XP05) protein transports pre-miRNA from the nucleus to the cytoplasm, and finally, the dicer enzyme aids in the formation of mature miRNA from the pre-miRNA. The first step in the canonical process is the production of the pri-miRNA transcript. RNA polymerase II/III participates in this process post-transcriptionally or co-transcriptionally once miRNA is attached to the RNA-induced silencing complex (RISC). The stem loop of pre-miRNA is disintegrated into 60-100 nucleotides by a microprocessor network. This 60-100 pre-miRNA is then synthesised into a 22 nucleotide long mature miRNA by dicer and RNase III. Duplex arrangement takes place after pre-miRNA evolves into mature miRNA in the presence of exportin5/RanGTP in the cytoplasm. An Ago family protein stacked in either a 5' or 3' orientation on mature miRNA.
Correspondingly, non-canonical miRNA biogenesis is divided into two categories: dicer-independent and Drosha/DGCR8-independent pathways. Some of the protein combinations associated with the non-canonical pathway include Drosha, Dicer, exportin, and Ago2. When the Drosha/Dgcr8 complex is unavailable, miRNA processing takes place in the nucleus. As a consequence, Dicer stimulates miRNA fabrication in the Drosha/DGCR8 sovereign mechanism, and miRNAs are bisected through Drosha (Saiyed et al., 2022)(Figure 1).
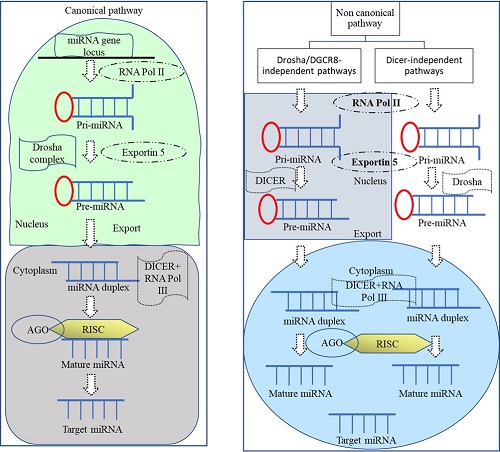
Figure 1 A schematic diagram of animal miRNA biogenesis.
The biosynthesis process of plant miRNA
Plant microRNAs (miRNAs) are made up of about 22 nucleotides. When compared to animal miRNAs, plant miRNAs have very little diversity in terms of regulation mechanisms and biosynthesis. Plant miRNAs are single-stranded hairpin precursors that emerge from distinct stem regions. For primary miRNAs (pri-miRNAs), the RNA polymerase II enzymes control the transcription process. Dicer-like 1 (DLC1) in nuclease converts these pri-miRNAs into stem-loop precursor miRNAs (pre-miRNAs). These pre-miRNAs were transported into the cytoplasm by HASTY, an exportin 5 homologue. Pre miRNA strands were converted to mature miRNA, and methyl groups were attached using a small RNA methyltransferase known as HUA Enhancer 1 (HEN1). Furthermore, AGO-containing RNA-induced silencing complex proteins that influence the guide miRNA strand were used to identify miRNA silencing. Plant miRNAs are distinguished from animal miRNAs by the presence of a 2′O-methyl group at their 3′ terminus(Achkar et al., 2016; Yu et al., 2017)(Figure 2).
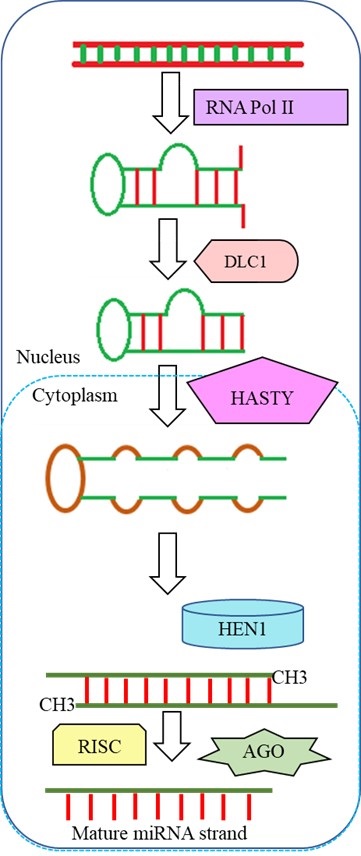
Figure 2 A schematic diagram of plant miRNA biogenesis.
Plant miRNA efflux and involvement in inter-species gene regulation
The modern paradigm explains how plant miRNAs efflux and consuming plants as a diet can transport and distribute them in the human body. A recent study found that these exogenous miRNAs were absorbed and indigestion of these miRNAs was accomplished in gastric glands with the help of SIDT1 and hit the target expression. Recent findings suggest that strawberry miRNAs can modulate autoimmune responses by regulating toll-like receptor 3 adherence and managing dendritic cell migration. In-vitro experiments also support the horizon gene transfer hypothesis, as a synthetic isoform of plant miRNA 171 can modulate the mTOR pathway in HEK293 cells by reducing the expression of the GNA12 signalling component. Additionally, in an in-vivo mouse model study, lettuce was used as a bioreactor to express two small silencing RNA sequence fragments that target the HBV surface antigen gene (HBsAg), and mice fed such lettuce for an extended period had less liver injury with non-toxic allergies (Chen et al., 2021). However, these findings were met with criticism and scepticism, with the main concerns focusing on the dependability and sensitivity of the techniques commonly used in the study of cross-kingdom miRNA transmission.
Plant miRNA adaptation as a novel disease controller
Endogenous miRNAs found in human cells are referred to as "cellular miRNAs," whereas those obtained from vegetable-based plant food are simply referred to as "plant miRNAs." Plant miRNA functions as a panacea in modern disease management. A substantial body of evidence supports the transfer of xenomiRNAs. Plant miRNA can regulate everything from cancer to diabetes and heart disease. The World Health Organization (WHO) reports that India currently has 1,324,413 cancer cases, with a projected 57.5 percent increase by 2040. COVID-19 has been confirmed in 528,275,339 people, with 6,293,414 deaths, according to WHO. Diabetes is the world's ninth most common disease, affecting around 422 million people globally. Furthermore, nearly 1.5 million deaths are directly attributable to diabetes, according to WHO research. According to the World Obesity Atlas 2022, one billion people will be obese worldwide by 2030, with one out of every five women and one out of every seven males being obese. In addition, WHO released a "Global action plan on physical activity 2018–2030: more active people for a healthier world" as part of this activity. Healthy diets and regular physical activity are promoted by WHO, and diabetes and obesity are expected to climb dramatically by 2025 globally. Furthermore, cardiovascular diseases kill 17 million people each year, accounting for 32 per cent of all deaths worldwide (WHO, 2022). Furthermore, all of these diseases are linked and on the rise as a result of a poor diet. According to Albert Einstein, "Nothing will benefit human health and increase the chances of life on Earth more than the transition to a vegetarian diet."
Plant miRNA in cancer prevention
According to studies, plant miRNA 159 can be associated with breast cancer progression and was found to reduce tumour growth in an in vivo mouse model. Evidence describes the interkingdom miRNA transfer in this study. miRNA 159 can transfer in humans with the help of extracellular vesicles (EVs), addressing the exosomes' conduction inside the human. These EVs can modulate cellular function exclusively in host cells (Chin et al., 2016; Teng et al., 2018). Recent research demonstrates that exosomes resembling nanoparticles from consumable plant parts can be associated with cancer pathways like Wnt-signalling, which is a critical regulator of cancer metastasis in mouse models (Mu et al., 2014). There is evidence that plant miRNA 159a and miRNA 156c affect the level of tumour necrosis factor (TNF) receptor superfamily member 1a (Tnfrsf1a) transcript, leading to downregulation of the TNF-signalling pathway and related inflammatory controllers packaging with exosome mediated nanovesicles (Aquilano et al., 2019). The study conducted by Li et al. supports the horizontal cross-kingdom interaction by stating that miRNA 167e-5p decreases intestinal cell proliferation by directing β-catenin mesenchymal marker. The miRNA from the extracts of Olea europaea has been shown to play a role in reducing the protein expression of has-miRNA 34a mRNA targets, reducing cell proliferation and inducing apoptosis in various in-vitro tumour models (Li et al., 2019; Minutolo et al., 2018).
The role of plant miRNAs in SARS-CoV2
In human-plant crosstalk, plant miRNA interacts with nucleic acids existing in human cells and modulates the human gene at a molecular level. Besides various roles in human disease management, plant miRNA additionally regulates the viral genome like SARS-Cov2 and influenza virus. Consumption of plant-derived miRNA 2911 from honeysuckle decoction (HD) feasibly slows down the viral replication and helps cure infected patients with SARS-coV2 (Zhou et al., 2020). Moreover, miRNAs from citrus sinensis (Orange), Prunus persica (Peaches), Vitis vinifera (Grapes) and Malus domestica (Apple) have a role in the viral genome. C. sinensis miRNA, specifically csi-miR169–3p, was discovered to play a role in the inhibition of SARS-CoV-2 and 772 Omicron variants worldwide. Moreover, miRNA from Zingiber officinale (Z. officinale) zof-miR2673b engages in silencing of viral genome RNA and accommodates the antiviral defence against SARS-CoV2 infection cross-kingdomly. A double-blind incidental study was carried out with Z. officinale and SARS-CoV2 patients and found that repeated consumption of Z. officinale tablets improved the clinical symptoms in patients (Mangukia et al., 2021, 2022; Safa et al., 2020). Furthermore, plant miRNA can be packed into edible nanoparticles and transported into humans. Six miRNAs from ginger and grapefruit were validated in the study, and their expression on SARS-CoV2 was confirmed by RT-PCR (Kalarikkal& Sundaram, 2021). According to the above scenario, plant-based miRNAs are frequently associated with anti-viral properties in the regulation of the mordent disease.
Plant miRNA in the Heart and Diabetes
The concept of diet influencing miRNA regulators for heart disease is relatively new. Consumption of resveratrol down-regulates myocardial ischemia and upregulates miRNA-20b and 21. It decreases VEGF levels. In line with this hypothesis, chitosan nano-partials were selected for the transportation of exog-miRNA 33 to reduce the cholesterol level in a xenograft mouse model, indicating that nano-particles carrying miRNA could be a subsequent approach for targeting atherosclerosis lesions (Collado et al., 2021). Dietary derived plant-based EVs may influence the human immune system by influencing the gut microbiota. For example, ginger exosomes similar to nanoparticles induced gut microbiota durability and profoundly affected Lactobacillus rhamnosus (LGG) gene expression in a mouse model. The LGG gene is well-known for its anti-diabetic and anti-obesity properties, as well as its ability to regulate gut microflora. It can also help with weight loss, leptin resistance, adiposity reduction, and improving glucose tolerance and insulin sensitivity. As a result, manipulating gut mutualistic LGG with dietary ginger exosome–like nanoparticles containing miRNAs could be a promising metabolic syndrome treatment strategy (Díez-Sainz et al., 2021)(Figure 3).
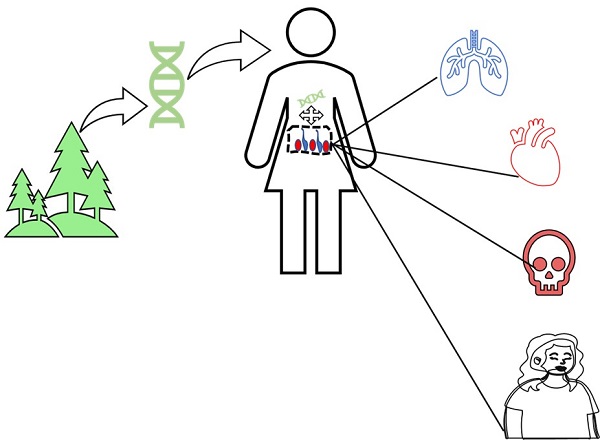
Figure 3 The Diagram of plant miRNA efflux and inter-species disease regulation transport. Figure depicts how plant miRNAs cross the human tissue barrier, travel through the strict human body environment, and influence gene mechanisms for various diseases.
Conclusion
In a nutshell, exploring the versatile role of plant-derived miRNAs in future therapies is expected to pave the way for future research and application of these newly identified, non-toxic, and low-cost plant active ingredients.
Acknowledgment
The authors sincerely acknowledge the Senior Research Fellowship Program File No. 2019-3856/CMB/BMS of the Indian Council of Medical Research.
References
Achkar, N. P., Cambiagno, D. A., &Manavella, P. A. (2016). miRNA Biogenesis: A Dynamic Pathway. In Trends in Plant Science (Vol. 21, Issue 12, pp. 1034–1044). Elsevier Ltd. https://doi.org/10.1016/j.tplants.2016.09.003
Aman, F., & Masood, S. (2020). How nutrition can help to fight against covid-19 pandemic. Pakistan Journal of Medical Sciences, 36(COVID19-S4). https://doi.org/10.12669/pjms.36.COVID19-S4.2776
Aquilano, K., Ceci, V., Gismondi, A., de Stefano, S., Iacovelli, F., Faraonio, R., di Marco, G., Poerio, N., Minutolo, A., Minopoli, G., Marcone, A., Fraziano, M., Tortolici, F., Sennato, S., Casciardi, S., Potestà, M., Bernardini, R., Mattei, M., Falconi, M., … Lettieri-Barbato, D. (2019). Adipocyte metabolism is improved by TNF receptor-targeting small RNAs identified from dried nuts. Communications Biology, 2(1). https://doi.org/10.1038/s42003-019-0563-7
Chaiwangyen, W. (2021). The Impact of Dietary Compounds in Functional Foods on MicroRNAs Expression. https://doi.org/https://doi.org/10.5772/intechopen.96746
Chen, X., Liu, L., Chu, Q., Sun, S., Wu, Y., Tong, Z., Fang, W., Timko, M. P., & Fan, L. (2021). Large-scale identification of extracellular plant miRNAs in mammals implicates their dietary intake. PLoS ONE, 16(9 September). https://doi.org/10.1371/JOURNAL.PONE.0257878
Chin, A. R., Fong, M. Y., Somlo, G., Wu, J., Swiderski, P., Wu, X., & Wang, S. E. (2016). Cross-kingdom inhibition of breast cancer growth by plant miR159. Cell Research, 26(2), 217–228. https://doi.org/10.1038/cr.2016.13
Collado, A., Jin, H., Pernow, J., & Zhou, Z. (2021). MicroRNA: A mediator of diet-induced cardiovascular protection. In Current Opinion in Pharmacology (Vol. 60, pp. 183–192). Elsevier Ltd. https://doi.org/10.1016/j.coph.2021.07.022
de Almeida, S. S. T., Horst, C. H., Soto-Sánchez, C., Fernandez, E., & de Almeida, R. T. (2018). Delivery of miRNA-Targeted oligonucleotides in the rat striatum by magnetofection with neuromag®. Molecules, 23(7). https://doi.org/10.3390/molecules23071825
Díez-Sainz, E., Milagro, F. I., Riezu-Boj, J. I., &Lorente-Cebrián, S. (2021). Effects of gut microbiota–derived extracellular vesicles on obesity and diabetes and their potential modulation through diet. Journal of Physiology and Biochemistry. https://doi.org/10.1007/s13105-021-00837-6
Kalarikkal, S. P., & Sundaram, G. M. (2021). Edible plant-derived exosomal microRNAs: Exploiting a cross-kingdom regulatory mechanism for targeting SARS-CoV-2. Toxicology and Applied Pharmacology, 414, 115425. https://doi.org/10.1016/J.TAAP.2021.115425
Kang, H. (2019). Molecular Sciences MicroRNA-Mediated Health-Promoting Effects of Phytochemicals. https://doi.org/10.3390/ijms20102535
Kura, B., Parikh, M., Slezak, J., & Pierce, G. N. (2019). molecules The Influence of Diet on MicroRNAs that Impact Cardiovascular Disease. https://doi.org/10.3390/molecules24081509
Li, M., Chen, T., He, J. J., Wu, J. H., Luo, J. Y., Ye, R. S., Xie, M. Y., Zhang, H. J., Zeng, B., Liu, J., Xi, Q. Y., Jiang, Q. Y., Sun, J. J., & Zhang, Y. L. (2019). Plant mir167e-5p inhibits enterocyte proliferation by targeting β-catenin. Cells, 8(11). https://doi.org/10.3390/cells8111385
Mangukia, N., Rao, P., Patel, K., Pandya, H., & Rawal, R. M. (2021). Identifying potential human and medicinal plant microRNAs against SARS-CoV-2 3′UTR region: A computational genomics assessment. Computers in Biology and Medicine, 136. https://doi.org/10.1016/j.compbiomed.2021.104662
Mangukia, N., Rao, P., Patel, K., Pandya, H., & Rawal, R. M. (2022). Unveiling the nature’s fruit basket to computationally identify Citrus sinensis csi-mir169–3p as a probable plant miRNA against Reference and Omicron SARS-CoV-2 genome. Computers in Biology and Medicine, 146, 105502. https://doi.org/10.1016/j.compbiomed.2022.105502
Minutolo, A., Potestà, M., Gismondi, A., Pirrò, S., Cirilli, M., Gattabria, F., Galgani, A., Sessa, L., Mattei, M., Canini, A., Muleo, R., Colizzi, V., & Montesano, C. (2018). Olea europaea small RNA with functional homology to human miR34a in cross-kingdom interaction of anti-tumoral response. Scientific Reports, 8(1). https://doi.org/10.1038/s41598-018-30718-w
Mu, J., Zhuang, X., Wang, Q., Jiang, H., Deng, Z. bin, Wang, B., Zhang, L., Kakar, S., Jun, Y., Miller, D., & Zhang, H. G. (2014). Interspecies communication between plant and mouse gut host cells through edible plant derived exosome-like nanoparticles. Molecular Nutrition and Food Research, 58(7), 1561–1573. https://doi.org/10.1002/mnfr.201300729
Safa, O., Hassaniazad, M., Farashahinejad, M., Davoodian, P., Dadvand, H., Hassanipour, S., &Fathalipour, M. (2020). Effects of Ginger on clinical manifestations and paraclinical features of patients with Severe Acute Respiratory Syndrome due to COVID-19: A structured summary of a study protocol for a randomized controlled trial. Trials, 21(1). https://doi.org/10.1186/s13063-020-04765-6
Saiyed, A. N., Vasavada, A. R., & Johar, S. R. K. (2022). Recent trends in miRNA therapeutics and the application of plant miRNA for prevention and treatment of human diseases. Future Journal of Pharmaceutical Sciences, 8(1), 24. https://doi.org/10.1186/S43094-022-00413-9
Seca, A. M. L., & Pinto, D. C. G. A. (2018). Plant secondary metabolites as anticancer agents: Successes in clinical trials and therapeutic application. In International Journal of Molecular Sciences (Vol. 19, Issue 1). MDPI AG. https://doi.org/10.3390/ijms19010263
Teng, Y., Ren, Y., Sayed, M., Hu, X., Lei, C., Kumar, A., Hutchins, E., Mu, J., Deng, Z., Luo, C., Sundaram, K., Sriwastva, M. K., Zhang, L., Hsieh, M., Reiman, R., Haribabu, B., Yan, J., Jala, V. R., Miller, D. M., … Zhang, H. G. (2018). Plant-Derived Exosomal MicroRNAs Shape the Gut Microbiota. Cell Host and Microbe, 24(5), 637-652.e8. https://doi.org/10.1016/j.chom.2018.10.001
WHO (World Health Organization). (2022, June). World Health Organization . https://www.who.int/
Yu, Y., Jia, T., & Chen, X. (2017). The ‘how’ and ‘where’ of plant microRNAs. In New Phytologist (Vol. 216, Issue 4, pp. 1002–1017). Blackwell Publishing Ltd. https://doi.org/10.1111/nph.14834
Zhou, L.-K., Zhou, Z., Jiang, X.-M., Zheng, Y., Chen, X., Fu, Z., Xiao, G., Zhang, C.-Y., Zhang, L.-K., & Yi, Y. (2020). Cell Discovery Absorbed plant MIR2911 in honeysuckle decoction inhibits SARS-CoV-2 replication and accelerates the negative conversion of infected patients. Cell Discovery, 6, 54. https://doi.org/10.1038/s41421-020-00197-3