Data-enriched edible pharmaceuticals (DEEP) is a novel pharmaceutical product design principle. DEEP has been proposed as a solution for personalized medicines, where each drug product, i.e., ‘every pill’, contains both the patient-tailored dose as well as information. This unique feature allows to easily identify the medicine, receive a customised information and trace the drug product by using a standard smartphone.
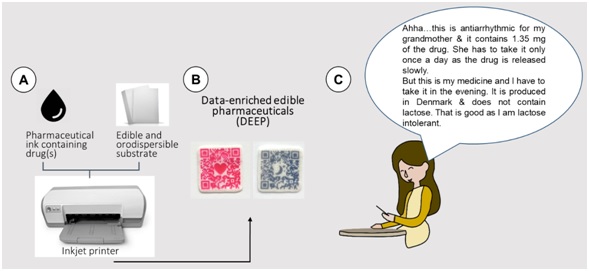
Figure 1: The concept of data-enriched edible pharmaceuticals from their fabrication (A,B) to the functionality to the patients (C).
Motivation for DEEP
Patient-tailored treatment can be a challenge with conventional product design principles, such as oral products based on tableting. There are several reasons for that. First of all, there is restricted flexibility in the dosage strengths that are available on the market. Administration of an inappropriate dose can lead to adverse drug reactions or lack of therapeutic effect. This is especially problematic if the therapeutic window of the drug is narrow. The absence of age-appropriate doses and formulations are particularly challenging for the paediatric and geriatric populations. Secondly, generic and complex information regarding the drug intake can lead to poorer compliance and/or incorrect use of the medicine. That is of special concern, if a patient has specific restriction in drug administration and/or takes multiple drugs. Finally, there are limited opportunities for tracing a single ‘pill’ back to the batch it belonged to.
Opportunities of DEEP
Data-enriched edible pharmaceuticals (DEEP) is a new drug product design principlethat (i) provide possibilities to produce flexible and accurate doses by digital manipulation of the drug product design. This is in contrast to the resource-consuming and tedious batch manufacturing that still dominates today; (ii) the unique appearance of DEEP helps to easily identify the medicine and to receive customised information (e.g., patient-specific administration instructions and videos) embedded in the QR code. This is accessed through scanning the QR code with a standard smartphone, (iii) making it possible to track and trace the drug product even if the original package is lost or not available[1–6]. Furthermore, DEEP technology paves the way towards the circular pharmaceutical supply chain by allowing the production of personalised medicines on-demand, avoiding accumulation of expired and tainted drug products. To add, the increasing use of wearable mobile devices, Internet of Things (IoT) based products, that could communicate with a standard smartphone and send signals about health status (e.g., pulse, oxygen saturation, etc) require drug products with the improved functionalities, such as DEEP.
How DEEP are fabricated
Fabrication of DEEP involves the following three main steps: (1) formulation of the printable ink that contains both the drug and edible colorant(s); (2) production of edible orodispersible substrate (e.g., ‘edible paper’) and (3) inkjet printing of the drug-containing ink onto the edible paper in the pattern of the QR code (Figure 1A, B).
The desired dose of the drug in DEEP can be achieved by digital adjustment of the QR code pattern that is imprinted. The dose can be digitally varied by (1) changing the physical size of the QR code pattern, in other words the bigger the size, the higher the dose is; (2) by modifying the pattern type of QR code and (3) by embedding the images (Figure 2).

Figure 2: Digital manipulation of the dose by modifying the QR code pattern and changing the colored pixel count: (A) dotted QR code pattern type; (B) original QR code pattern type; (C) QR code pattern with embedded image; and (D) the increased physical size of the printed QR code. The scale bar is 2 cm.
The release of drug(s) from DEEP can be modified (for example, slow down) by applying the polymer coating.
Patient involvement in product design
Patient involvement in the drug product design can improve compliance and adherence to the medication. DEEP presented in Figure 2 were fabricated based on the patients’ preferences in regards to color, size and design. Patients that were interviewed about DEEP were positive towards this new dosage form and preferred DEEP in either blue or red color with a size that would not be bigger than the tongue.
Limitations of DEEP technology
Until now, DEEP technology is more suitable for low dose drug products due to the limited absorption and soaking capacity of the ‘edible paper’,which does not allow printing a high volume of the ink. In addition, printing of higher volume of the ink would require longer fabrication time.
References
1. Edinger, M.; Bar-Shalom, D.; Sandler, N.; Rantanen, J.; Genina, N. QR encoded smart oral dosage forms by inkjet printing. Int. J. Pharm.2018, 536, 138–145, doi:10.1016/j.ijpharm.2017.11.052.
2. Fastø, M.M.; Genina, N.; Kaae, S.; Kälvemark Sporrong, S. Perceptions, preferences and acceptability of patient designed 3D printed medicine by polypharmacy patients: a pilot study. Int. J. Clin. Pharm.2019, 41, doi:10.1007/s11096-019-00892-6.
3. Öblom, H.; Cornett, C.; Bøtker, J.; Frokjaer, S.; Hansen, H.; Rades, T.; Rantanen, J.; Genina, N. Data-enriched edible pharmaceuticals (DEEP) of medical cannabis by inkjet printing. Int. J. Pharm.2020, 589, 119866, doi:10.1016/j.ijpharm.2020.119866.
4. Nørfeldt, L.; Bøtker, J.; Edinger, M.; Genina, N.; Rantanen, J. Cryptopharmaceuticals: Increasing the Safety of Medication by a Blockchain of Pharmaceutical Products. J. Pharm. Sci.2019, 108, doi:10.1016/j.xphs.2019.04.025.
5. Raijada, D.; Wac, K.; Greisen, E.; Rantanen, J.; Genina, N. Integration of personalized drug delivery systems into digital health. Adv. Drug Deliv. Rev.2021, 113857, doi:10.1016/J.ADDR.2021.113857.
6. Chao, M.; Öblom, H.; Cornett, C.; Bøtker, J.; Rantanen, J.; Sporrong, S.K.; Genina, N.; Torrado, J.J. Data-Enriched Edible Pharmaceuticals (DEEP) with Bespoke Design, Dose and Drug Release. Pharm. 2021, Vol. 13, Page 18662021, 13, 1866, doi:10.3390/PHARMACEUTICS13111866.