A broad spectrum of preclinical and clinical studies is required for the success of the drug development process. Human physiology relevant experiment setups can boost drug discovery. Therefore, this article outlines the recently developed organ-on-a-chip technology and its benefits in the drug development pipeline and its future perspectives.
The drug development process is highly complex, time-consuming, costly and labour intensive. There are many steps involved in developing the novel therapeutics, including target discovery and validation, lead compound identification and optimisation, preclinical studies, clinical trials, and finally, the FDA review and approval. Even after the development and approval of novel therapeutics, it requires a spectrum of the post-approval monitoring process. Out of the mentioned stages of drug development, preclinical studies and clinical trials require a preponderance of time and money. A comprehensive and considerable amount of in-vitro studies and animal testing is necessary to rule out and predict the side effects during human trials or consumption. The genetic difference, cross-species variation, and physiological differences between human and other in-vivo animal models may result in the exhibition of unpredicted toxicity during the human trials even though it qualifies the preclinical trials on the animal model. Therefore, alternative models that mimic the microcellular niche, physiological conditions, and disease progression in humans are necessary. More importantly, animal testing is often associated with an ethical dilemma. Three-dimensional models, organoids and organ-on-a-chips (organ chips) models have gained considerable momentum in replacing the animal models. However, the in-vivo animal models are still considered the gold standard in many testing and research and development experiments. Out of the alternative system developed in recent years, organ-on-a-chip technology stands out due to its unique ability to incorporate various human physiology relevant cues. Organoids can be used to generate complex tissue architecture. Combining organoid and organ-on-a-chip technologies can therefore provide a paradigm shift in the drug development pipeline.
What is an "Organ-on-a-chip" platform?
Organ-on-a-chips are microfluidic chips containing living human cells that reconstitute various organ-level functions. These micro-engineered chips can recreate the tissue-tissue interface and endothelium lined blood vessels (Fig 1), provide the respective mechanical force required for relevant physiology and the controlled fluid flow through the channel. The fluid flow enables the spatiotemporal gradient of chemicals, continuous supply of nutrients and removal of wastes and permits the real-time monitoring of various parameters. Organ-on-a-chip allows the users to study different biochemical reactions occurring at the tissue-tissue interface, including transport, permeability, immune cells recruitment, absorption and conductivity. The ability to perform the incorporation of the microbiome is an added advantage of this technology. Various such organ-on-a-chip models can be coupled together through the vascular channel to develop the multi organ-on-a-chip models.
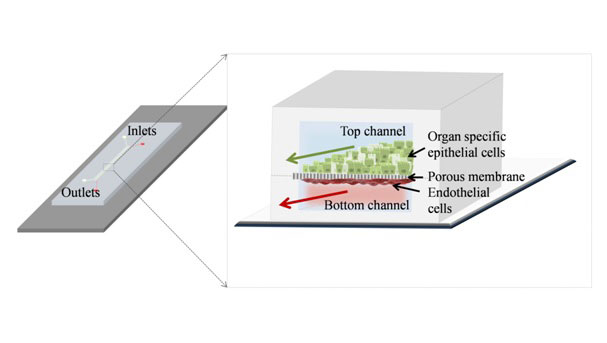
Figure 1. Schematic representation of an organ-on-a-chip model consisting of organ-specific cells and endothelial cells cultured on opposite sides of a porous membrane under dynamic flow conditions.
Voyage of "Organ-on-a-chip" Technology
A variety of organ-on-a-chips have been developed and improvised to reconstruct specific organ-level functions. The lung-on-a-chip developed by Dr Donald E. Ingber’s group from the Wyss Institute, Harvard University, created a revolution in organ chip’s biologically inspired design and microengineering. They have successfully recreated a functional alveolar-capillary interface with cyclic mechanical strain by applying vacuum and demonstrated the immune cell transmigration and cytokine production involved in the infection and inflammation while challenging with bacteria and nanoparticles [1]. The fabricated lung-on-a-chip was later used for modelling chronic obstructive pulmonary disorder, predicting drug concentrations, influenza virus infection, cystic fibrosis and most recently for modelling the SARS-CoV-2 viral entry, replication, host response, immune cells response, and validating the repurpose therapeutics for COVID-19. The Organ chip platforms demonstrated that the co-administration of anticoagulant drug nafamostat with the oseltamivir doubled the therapeutic time window and inhibited the infection of pseudo-typed SARS-CoV-2 virus while administrated with the amodiaquine in a clinically relevant dose [2].
Organ-on-a-chip in toxicity assessments
Due to being the primary site of drug and toxin metabolism, the hepatic system is a major area in drug development. Drug withdrawal from the market can occur due to the unpredicted drug-induced liver injury (DILI). Careful monitoring and proper preclinical studies are required to assess the molecule's hepatotoxicity and avoid potential acute and chronic liver injuries. The organ chips of the liver model can maintain the cellular microenvironment and enable long-term repeated drug toxicity experiments. Spheroid culture, co-culture methods can incorporate into the organ chip to reconstitute the organ-level function of the liver. The complex multi-cellular arrangement of the liver tissue is required to model functional liver models. The cell patterning in the liver chip can be achieved through various techniques such as field-induced dielectrophoresis [3], micro-fences and by manipulating the laminar flow conditions [4]. Co-culture of parenchymal and nonparenchymal cells, including Kupffer cells, stellate cells, and endothelial cells, enables signal transduction of molecule, enhancing the liver function and maintaining the culture long-term. Various research groups have utilised the liver-on-a-chip devices to assess the hepatotoxicity of drug molecules, cross-species toxicity of hepato-toxicants [5], hepatoprotective activity [4], and model various disease conditions such as liver fibrosis, alcoholic and non-alcoholic fatty liver conditions [6]. Additionally, biosensors integrated chip can provide the dynamics of the liver damage by monitoring different parameters such as the mitochondrial function, biomarkers secreted by the cells, changes in glucose and lactose levels etc. Higher sensitivity, reliability, accuracy, minimal reagent volume, and ability to assess the dynamics make the liver-on-a-chip a promising alternate for evaluating hepatotoxicity of novel therapeutics.
The kidney is another organ of interest in the drug development pipeline due to its vital role in drug excretion and fluid homeostasis. Drug-induced toxicity can decrease kidney function predominantly. The complex and vascularised structure is required to model the drug-induced nephrotoxicity. Conventional static cell culture or the in vivo animal models cannot predict the nephrotoxicity of the efficacy of drug molecules completely. However, the micro-physiological system of the kidney model can deliver a more realistic response than the conventional methods and provide better results. Recently various types of kidney-on-a-chip devices have been developed to study acute kidney injury, drug-induced nephrotoxicity, drug interaction and to assess the drug effects [7]. The renal tubular cells continuously experience shear stress due to the fluid flow, and the shear stress is essential for cytoskeleton organisation, junctional reformation and regulation of ions concentrations. The inherent features of the microfluidic chip, such as the incorporation of fluid flow through peristaltic or pressure pumps, enables precise control over the fluid flow rate and thereby the shear stress. The controllable development of bio-mimetic kidney system through the incorporation of membranes, hydrogels, extracellular matrixes and phase guides can reproduce the three-dimensional microenvironment.
The oral bioavailability and absorption of small molecules and drug compounds can investigate through the gut/intestine-on-a-chip platforms. Recently de Haan et al. developed a compartmentalised gut-on-a-chip platform consisting of mouth, stomach and intestine chips connected through the fluidic path to study the bio-accessibility of small molecules [8]. The device has enabled the recapitulation of various chemical and enzymatic breakdowns occurring in the mouth, stomach and intestine. The bioavailability of drug molecule is an essential factor determining the fraction of molecule of interest enters the circulatory system and finally reaches the target sites. The gut-on-a-chip can be used to study pathophysiology, toxicity, microbiome interaction occurring in intestines. The formation of villus-crypt structure and mucus production has been demonstrated in continuously perfused chips under cyclic mechanic forces to mimic the peristaltic forces experiencing in the intestine. Investigation of gut microbiome-epithelial interaction is necessary to study physiology and disease progression occurring in the intestine. However, most of the microbiome colonising in the human intestine does not exist in mice. Intestine chips can enable toxicity studies or physiology studies and emulate human epithelium-microbes interplay and homeostasis occurring in the intestines. The drug interaction can be monitored in intestine-on-chip by permeability studies, barrier integrity analysis, enzymatic activity and effluent composition studies. Proper implementation of these chips hence reduces the cost of drug development considerably and provides better data.
Concept of the state of art ‘Human-on-a-chip’ technology
Multi-organ chips can emulate systemic response. A multi-organ chip consists of cells from various organs connected through the vascular network. These systems are beneficial in assessing the off-target toxicity and model the pharmacokinetics and pharmacodynamics (PK-PD) associated with a candidate molecule.
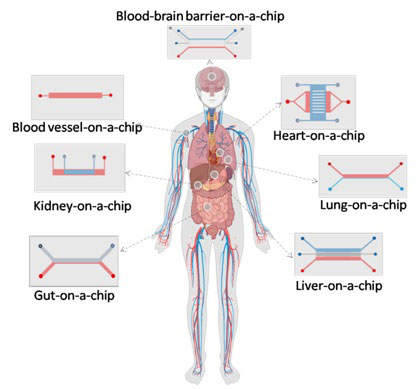
Figure 2 Schematics various models of Organ-on-chip devices
Various single and multi-organ-on-a-chips (Fig. 2) have been developed to model the first-pass metabolism, biotransformation, metabolism drug molecule and its metabolites. For example, a liver-kidney chip is used to predict the aristocholic acid's (AA-1) renal toxicity on humans and demonstrated the metabolism of AA-1 in liver cells escalated the cytotoxicity in kidney cells [9]. Recently, more complex multi-organ chips were developed for investigating the toxicity response of drug molecules. The multi-organ chip designed by Dr Michael L Schuler’s group consisting of heart, liver, muscle and neurons modules enabled a long-term culture up to 14 days. It is used to investigate the toxicity response under continuous flow conditions [10]. Dr Shuler's group increased the complexity of studying the off-target toxicity associated with the anticancer therapeutics by including the cantilevers, microelectrodes arrays, and different cancer cell lines [11, 12]. Many such organ-on-a-chip devices can be combined to develop human-on-a-chip devices ultimately. The pumpless multi-compartment model of multi-organ chip from Dr Shuler's group, which houses 13 different organs, was a step toward the human-on-a-chip concept [13]. The device accommodated various barrier cell lines and non-barrier cell lines, and the cells were viable and functional for up to seven days. The vascularised organs coupled via the fluidic network from the Dr Ingber group have demonstrated the quantitative prediction of PK-PD of drug molecules [14]. The PK-PD of cisplatin analysed by using the fluidically coupled organ chips was matched with the patient data. Although human-on-a-chip can reduce the animal tests during drug development and the ability to perform various toxicity studies, it is associated with various challenges. The proliferation rates of cells from various organs are different, and common media is required while connecting various organs, and the proper scaling of organ size and cell number is necessary to obtain a physiologically relevant PK-PD model.
Future perspectives
Human on a chip technology will revolutionise the field of drug development in the near future. The careful implementation of human on a chip technology will lead to the possible acceleration of the drug development process and animal model replacement [15]. Moreover, with the use of patient-derived cells in the devices, human-on-a-chip technology might lay the foundation for the future of personalised medicine. The human-on-a-chip technology can deliver various readouts to access the physiological conditions of the cells. However, to obtain a physiology relevant model and a meaningful PK-PD model, proper scaling is required [16,17]. With no doubt, organ-on-chip technology can be the next generation technology having a broad spectrum of applications in drug development, personalised medicines, rare disease modelling and so on.
References
1. Huh D, Matthews BD, Mammoto A, Montoya-Zavala M, Hsin HY, Ingber DE. Reconstituting organ-level lung functions on a chip. Science. 2010 Jun 25; 328(5986):1662-8.
2. Si L, Bai H, Rodas M, Cao W, Oh CY, Jiang A, Moller R, Hoagland D, Oishi K, Horiuchi S, Uhl S. A human-airway-on-a-chip for the rapid identification of candidate antiviral therapeutics and prophylactics. Nature Biomedical Engineering. 2021 May 3:1-5.
3. Ho CT, Lin RZ, Chen RJ, Chin CK, Gong SE, Chang HY, Peng HL, Hsu L, Yew TR, Chang SF, Liu CH. Liver-cell patterning lab chip: mimicking the morphology of liver lobule tissue. Lab on a Chip. 2013; 13(18):3578-87.
4. Deng J, Cong Y, Han X, Wei W, Lu Y, Liu T, Zhao W, Lin B, Luo Y, Zhang X. A liver-on-a-chip for hepatoprotective activity assessment. Biomicrofluidics. 2020 Nov 30; 14(6):064107.
5. Jang KJ, Otieno MA, Ronxhi J, Lim HK, Ewart L, Kodella KR, Petropolis DB, Kulkarni G, Rubins JE, Conegliano D, Nawroth J. Reproducing human and cross-species drug toxicities using a Liver-Chip. Science translational medicine. 2019 Nov 6;11(517).
6. Hassan S, Sebastian S, Maharjan S, Lesha A, Carpenter AM, Liu X, Xie X, Livermore C, Zhang YS, Zarrinpar A. Liver‐on‐a‐Chip Models of Fatty Liver Disease. Hepatology. 2020 Feb;71(2):733-40.
7. Cohen A, Ioannidis K, Ehrlich A, Regenbaum S, Cohen M, Ayyash M, Tikva SS, Nahmias Y. Mechanism and reversal of drug-induced nephrotoxicity on a chip. Science Translational Medicine. 2021 Feb 24;13(582).
8. De Haan P, Santbergen MJ, van der Zande M, Bouwmeester H, Nielen MW, Verpoorte E. A versatile, compartmentalised gut-on-a-chip system for pharmacological and toxicological analyses. Scientific reports. 2021 Mar 1;11(1):1-3.
9. Chang SY, Weber EJ, Sidorenko VS, Chapron A, Yeung CK, Gao C, Mao Q, Shen D, Wang J, Rosenquist TA, Dickman KG. Human liver-kidney model elucidates the mechanisms of aristolochic acid nephrotoxicity. JCI insight. 2017 Nov 16;2(22).
10. Oleaga C, Bernabini C, Smith AS, Srinivasan B, Jackson M, McLamb W, Platt V, Bridges R, Cai Y, Santhanam N, Berry B. Multi-organ toxicity demonstration in a functional human in vitro system composed of four organs. Scientific reports. 2016 Feb 3;6(1):1-7.
11. McAleer CW, Pointon A, Long CJ, Brighton RL, Wilkin BD, Bridges LR, Sriram NN, Fabre K, McDougall R, Muse VP, Mettetal JT. On the potential of in vitro organ-chip models to define temporal pharmacokinetic-pharmacodynamic relationships. Scientific reports. 2019 Jul 3;9(1):1-4.
12. McAleer CW, Long CJ, Elbrecht D, Sasserath T, Bridges LR, Rumsey JW, Martin C, Schnepper M, Wang Y, Schuler F, Roth AB. Multi-organ system for the evaluation of efficacy and off-target toxicity of anticancer therapeutics. Science translational medicine. 2019 Jun 19;11(497).
13. Miller PG, Shuler ML. Design and demonstration of a pumpless 14 compartment microphysiological system. Biotechnology and bioengineering. 2016 Oct;113(10):2213-27.
14. Herland A, Maoz BM, Das D, Somayaji MR, Prantil-Baun R, Novak R, Cronce M, Huffstater T, Jeanty SS, Ingram M, Chalkiadaki A. Quantitative prediction of human drug pharmacokinetic responses using multiple vascularized organ chips coupled by fluid transfer. Nature biomedical engineering. 2020 Apr;4(4):421.
15. Mohanan PV. Futuristic Applications of Multi-Organ-on-a-Chip/Human-on-a-Chip in Biomedical Science. ECPharmacology and Toxicology 2019 ECO.02: 01-02.
16. Syama S, Mohanan PV. Microfluidic based human-on-a-chip: A revolutionary technology in scientific research. Trends in Food Science & Technology. 2021 Feb 110: 711-728.
17. Joseph X, Akhil V, Arathi A, Mohanan PV. Comprehensive development in organ-on-a-chip technology. Journal of Pharmaceutical Sciences. 2021 Jul 26. DOI: 10.1016/j.xphs.2021.07.014