As many as four out of ten drugs identified as potential lead candidates during lead optimisation stage, fail in late stage of clinical trials. Failures of these late stage leads are one of the primary causes for the shrinking drug pipeline resulting in higher cost pressures due to waste of significant capital investment right at early stage Research. Recent drug-approval reports from the FDA’s website shows only 21 new drugs approved in 2010, down from 25 in 2009 and 24 in 2008. This trend clearly designates the dramatic shift in drug approvals from NMEs (New Molecular Entities) addressing common and easy to treat diseases over to NMEs addressing unmet health and medical needs and targeting complex and unusual diseases. Also, this is setting tremendous pressure on Pharmacos to shift their focus and investments on improving productivity & innovation across Pharmaceutical Research & Development (R&D) to ensure that only quality candidates get entry for further clinical development.
Even though productivity crises have been seen almost for more than a decade now, with numerous attempts by pharmacos to bring the productivity level back on track by experimenting new R&D models (e.g. Novartis changing its focus to encourage innovation by targeting areas of greater patient needs; Pfizer partnering with leading academic institutions to promote innovation; and GSK focusing on productive R&D functions that encourage creativity and facilitate accelerated discovery and development of new medicines to strengthen the pipeline). Most Pharmacos are exploring emerging markets for outsourcing some of these key R&D activities. However, the fact still remains - no great success in this space so far. The studies have also revealed that this decline is mainly associated with increasing R&D investments in areas of higher risk or maximum probability for failure which correspond to unmet therapeutic needs and unexploited biological mechanisms.
Looking at the associated cause, R&D productivity improvement can be approached in three ways.
The following article describes the stage gate framework comprising of holistic phase-review designed for New Product Development (NPD) system and how it can be used to show significant productivity improvement if implemented & applied successfully as part of overall effective project management. The stage gate framework is a series of systematic, constructive and cross functional reviews providing increased scrutiny over R&D projects, where the gates refer to key decision points at each important milestone of the project to assess: what has been achieved, whether it is sufficient, and based on the information available if the project can be sanctioned to the next stage. If at any point of time in the process the management team has any questions, concerns or there is any vagueness around the availability of scientific information, they can stop the project moving ahead to next stage or abandon it completely thereby resulting in subsequent savings in terms of money, resources and efforts on the later stages of development. Thus the stage gate framework provides very strategic and important “Go/No Go” decisions for Research & Development (R&D) projects with consensus from all the important business stakeholders and helps establish criteria for:
Is Adoption in Pharma still a challenge?
The stage gate framework is well adopted by most non-pharma companies like P&G , 3M and Corning, but adoption in pharmaceutical space has yet to pick up speed due to lack of alignment of process and technology with stage gate model. This challenge is difficult but not impossible to overcome. Hence, this has been proven successful through detailed assessment of existing capabilities to lay down a roadmap for developing a right combination of effective tools and capabilities along with well-designed stage gate framework in a typical R&D set up.
How one should adopt the stage gate methodology?
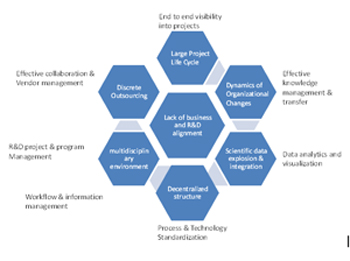 The detail capability assessment will help one to identify the process and technology gaps, prioritization of capabilities to be built followed by business approval and detail requirement gathering of the new capabilities. This will result in an effective implementation of the stage gate framework with assurances that other associated challenges in terms of process and technology have been addressed, so the stage gate model can work successfully and continuous performance improvement can be tracked.
The detail capability assessment will help one to identify the process and technology gaps, prioritization of capabilities to be built followed by business approval and detail requirement gathering of the new capabilities. This will result in an effective implementation of the stage gate framework with assurances that other associated challenges in terms of process and technology have been addressed, so the stage gate model can work successfully and continuous performance improvement can be tracked.
The effective knowledge and information management tools will address the flow of scientific information and data throughout the long research cycles, thereby enabling effective transfer of knowledge to a different set of established business users in case of organizational changes while ensuring the continuity in information. Best in class data integration techniques are must for seamless integration of the scientific data generated across multiple projects and from disparate systems & data sources, followed by archival in the centralized database. The latency of the information in the consolidated data store will depend upon whether batch or the real time data uploads are being applied to the data store providing real time information available to the scientists. Advanced business intelligence & data analytics solutions will offer abstraction of the data in the multiple formats and views to enable actionable insight, predictive analysis to reach the statistical conclusion much faster.
This will ensure that huge amount of unstructured scientific data getting generated, makes sense to other stakeholders of even the nonscientific community.
Implementation of best program and project management methodologies will offer end to end visibility in to various projects while maintaining synergy across multiple Research & Development (R&D) programs running in parallel. This will result in better project planning and risk mitigation strategy for project timelines, inter-dependency of projects, resource allocations, budgets and research outcomes. Collaborative portals will be an added benefit to enable brainstorming discussions and decision making in the multi –disciplinary environment to address Logistics, Intellectual property, Regulatory Compliance and Legal concerns well ahead of time. Use of standard and best practices always will confirm success in diverse geographic spread of R&D operations.
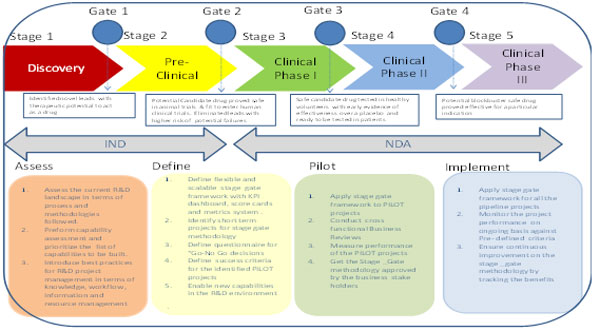
The right blend of these key capabilities will improve visibility and decision making across R&D projects at each stage and sub stage with the key questions answered in terms of risks and benefits of the project status of important check points and success criteria of the project to progress to the next stage of predefined activities & deliverables. This will facilitate the easy adoption of stage gate methodology among business users and easy sustenance for continuous tracking of the benefits in terms of improved R&D productivity and increased focus on innovation to drive growth.
The ideal approach to implement the stage gate methodology should follow the sequence of activities such as Assess, Plan, PILOT and Go Live.
Conclusion:
The majority of Pharma companies are already on a fast track towards the R&D productivity improvements initiatives, but many have yet to discover the real formula for success. If they are really serious about this issue, then exploring a historically well proven and well established stage gate framework combined with effective tools and processes is a worthwhile investment to explore.