Recently, FPS and a major European manufacturer of API (Active Pharmaceutical Ingredient) had to face a challenging project concerning the micronisation process of a specific product. In our R&D and Test Centre some trials have been performed in order to obtain the requested performance in term of final PSD (Particle Size Distribution) and productivity. Our process specialist, in collaboration with the customer, studied and tested in detail the geometric and process parameters to maximize the requested results.
FPS is an Italian company specialized in the production of containment and micronisation systems for Pharmaceutical, Biotech and Fine Chemical companies.
Over the past 18 years, FPS has grown to become the world's leading manufacturer of High Contaimentinsulators for fine powders with over 1,000 systems installed.FPS global technical team supports these systems in the 40 countries where they are installed.
Recently, FPS and a major European manufacturer of APIs (Active Pharmaceutical Ingredients) had to face a challenging project concerning the Micronisation process of a specific product. In our R&D and Test Centre trials have been performed in order to obtain the requested performance in term of final PSD (Particle Size Distribution) and productivity. Our process specialist, in collaboration with the customer, studied and tested in detail the geometric and process parameters to maximize the requested results.
The requested system is designed considering four different steps: product charging, product micronising, separation of product from process gas, product discharging and packing-off system.
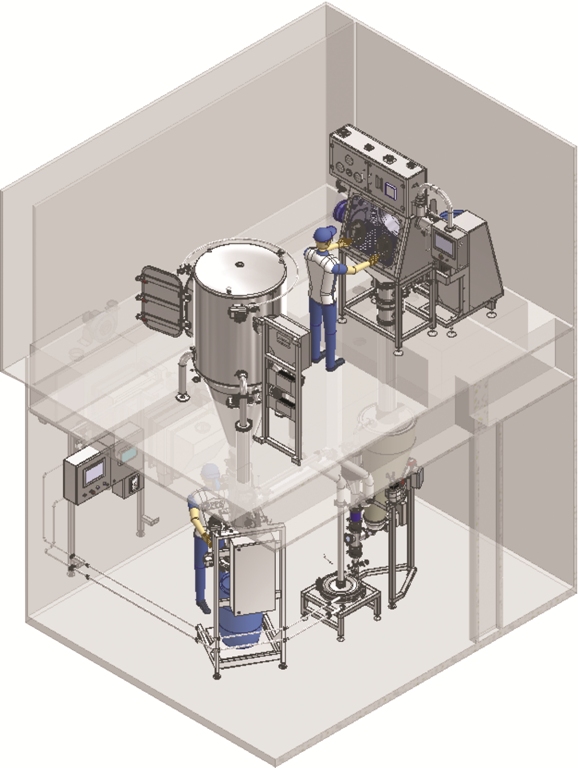
The product is charged in an isolator that is installed on the first floor. An automated drum lifting and turning device is provided to connect the drum, that contains the product, to the isolator for powder discharging. Inside the isolator the bag is opened and the product is transported into the dosing hopper, at ground floor, by gravity. In order to achieve better control of the dosing phase a gravimetric dosing unit is installed. Micronized product is transported through a dedicated line to the separation cyclone filter installed on the first floor to the side of the charging isolator. Micronized powder is recovered and discharged by means of a dedicated pack-off discharging system completed with an automated weighing system.
The FPS Sales Manager and micronisation expert, Dr. SamueleBissola, says: “The success of this challenge was certainly due to the experience gained in similar projects and the ability to combine two technologies in one system, maintaining high quality level and safety standard “.
SAVE THE DATES
If you would like to examine containment issues in depth, don't miss the chance to participate to the next workshops:
• May 30th | at the end of 3rdAnnual HPAPI Medicines Conference 2019 (Milan) | FPS production plant in Fiorenzuolad’Arda (PC).
• October 4th | at the end of 5th Annual HPAPI Summit (Rome) | Rome
Contact us to get a special discount &additional info: [email protected]