Viral infections are a serious public health issue, necessitating the development of innovative treatment methods. Cellular kinases are one of the most significant host resources that mediate the virus's life cycle at various stages (entry, replication, budding, and release). The repurposing of FDA-approved kinase inhibitors with antiviral properties is discussed in this article.
The emergence of virus infections is a serious issue, and the development of broadly acting antivirals has heightened concern for future viral epidemics. The majority of these drugs operate directly on the proteins encoded by the individual viral genome. However, they have the following disadvantages: (i) limited treatment coverage, which does not address the huge clinical requirements that arise with the development of novel viruses, and (ii) fast emergence of virus-induced drug resistance.
An alternate strategy aimed at the host-based objectives should be used to overcome this obstacle. Viruses multiply by interacting with a wide range of host cellular proteins. A growing body of evidence shows that viruses hijack the host cellular machinery soon after they enter the cells to get assistance in completing the replication life cycle.
Cellular kinases are one of the most important host cellular resources for enveloped viruses at various phases of their life cycle. They regulate cell proliferation, cell cycle control, apoptosis, inflammation, and metabolism, among other biological activities. Although kinases are well-known as cancer targets, they have recently garnered interest as potential therapeutic targets for antiviral treatment development. Targeting host cellular kinases as a therapeutic strategy has several benefits over traditional, direct-acting antiviral agents. Kinase-targeted treatments provide a higher level of resistance protection and cover a larger range of viral genotypes or serotypes, resulting in a broad spectrum of efficacy. Indeed, several research articles reported the involvement of kinases as potential mediators in the life cycle of various viral infections.
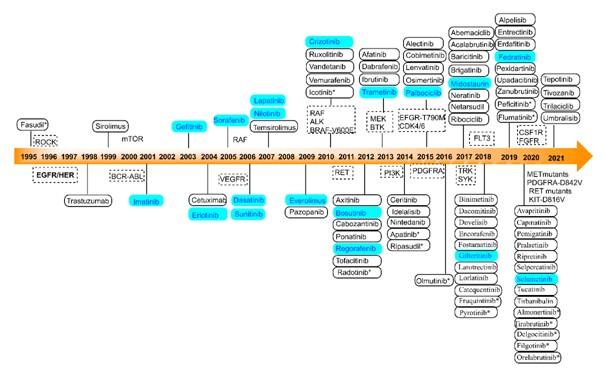
Figure 1. Timeline of approved kinase inhibitors. The inhibitors mentioned in blue are reported to possess antiviral activity at least in the in vitro system. The FDA has approved 71 SMKIs; China's National Medical Products Administration (NMPA) has approved eight more; Japan's Pharmaceuticals and Medical Devices Agency (PMDA) has approved five; the European Medicines Agency (EMA) has approved one; and South Korea's Ministry of Food and Drug Safety (MFDS) has approved two (all non-FDA approvals are indicated by an asterisk).
Numerous attempts have been made by academia and industry to produce ligands (agonists and antagonists) that control the activity of kinases due to their crucial physiological significance. The number of authorised kinase inhibitors has risen to 98 since the initial approval of Fasudil in 1995 (Fig. 1). The FDA has authorised a total of 71 small molecule kinase inhibitors (SMKIs) that target 21 distinct kinase families, which make up 20% of the human kinome. More than 20 of the 71 SMKIs have been repurposed for antiviral activity in addition to their established medicinal uses. The repurposing of FDA-approved medicines having broad-spectrum antiviral activity is discussed in this article (see representative examples in Figure 2).
AXL receptor tyrosine kinase inhibitors. The involvement of receptor tyrosine kinase AXL as a viral entry factor/immune modulator has been established in the dengue, Zika, and Ebola viruses. The ZIKA virus (ZIKV) penetrates human glial cells expressing the AXL receptor via clathrin-mediated endocytosis. SARS-CoV-2 endocytosis, which is the source of the current COVID-19 outbreak, has been linked to AXL's interaction with spike protein on the host cell membrane. SARS-CoV-2 viral entry into pulmonary and bronchial epithelial cells was dramatically reduced when AXL was knocked down in H1299 and A549 cells, indicating that AXL is needed for virus entry.
The approved FLT3/AXL inhibitor Gilteritinib has been shown to prevent SARS-CoV-2 replication in Huh7 cells (EC50 225 nM). Bemcentinib, an investigational Axl-targeting drug, was demonstrated in clinical studies to block SARS-CoV-2 entry, suggesting that it might be beneficial in the treatment of early SARS-CoV-2 infection.
The epidermal growth factor receptor (EGFR) inhibitors. EGFR is a member of the ErbB subfamily of RTKs and has been implicated in the infection of unrelated viruses. EGFR is required for HCV viral endocytosis. The EGFR pathway has been associated with vaccinia virus transmission and mortality, as well as the entry mechanism for influenza A, HCV, and Epstein-Barr virus. IAV and other viruses have been shown to exploit EGFR to evade the host's immune system.
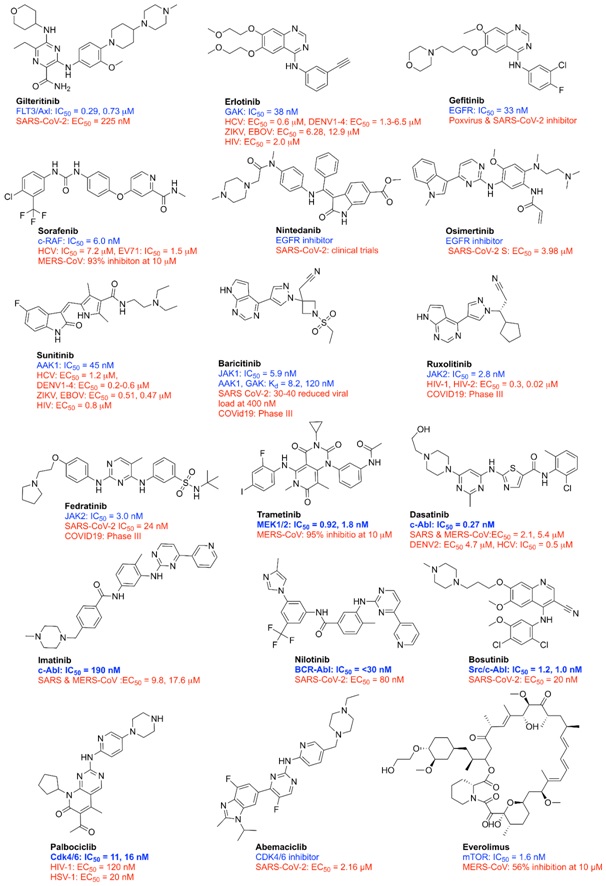
Figure 2. FDA approved different kinase inhibitors with antiviral properties. Kinase inhibitory activity (blue) and antiviral activity (red).
Erlotinib, an FDA-approved EGFR inhibitor used to treat a variety of cancers, has previously been shown to decrease HCV entrance (IC50 0.45 µM) and infectivity in Huh7 cells (EC50 0.53 µM). Gefitinib, an FDA-approved EGFR inhibitor, stops the poxvirus from spreading by inhibiting EGFR.
In a variety of human coronavirus infections, including SARS-CoV-2, EGFR inhibitors such as Erlotinib or Gefitinib, as well as the PDGFR inhibitor Sorafenib, have lately been advised to prevent an excessive fibrotic response. However, there is no obvious link between these kinase inhibitors' antiviral efficacy and antiviral activity.Nintedanib, an FGFR inhibitor, is now being tested in clinical studies to treat moderate COVID-19 pulmonary fibrosis patients (NCT04338802). Despite the cytotoxicity of Osimertinib, the EGFR inhibitor has been licensed for the treatment of NSCLC, and recently been identified as a possible SARS-CoV-2 S protein inhibitor, with an EC50 of 3.98 M and the ability to rescue 60% of the cytopathogenic impact of SARS-CoV-2.
Adaptor-associated kinase 1 (AAK1), and cyclin G-associated kinase (GAK) inhibitors. AAK1 and GAK regulate different steps in the life cycle (entry, assembly, and release) of viruses such as Ebola, dengue, HCV, and SARS-CoV-1 and -2, and MERS-CoV. Most viruses enter host cells by receptor-mediated endocytosis, which is controlled by AAK1 and CGAK1. The virus is considered to be prevented from infecting host cells by inhibiting these two kinases. Hepatitis C virus (HCV) entrance is also controlled by the proteins AAK1 and GAK. The significance of AAK1 and GAK in viral infection has also been extended to viruses other than HCV, such as DENV and EBOV.
Sunitinib, which inhibits AAK1 and GAK, has antiviral efficacy against DENV and EBOV. Sunitinib and Erlotinib were discovered to inhibit viral infection by inhibiting AP-mediated intracellular membrane trafficking via AAK1 and GAK at the molecular level. In Huh-7.5 cells, Sunitinib inhibited the HCV infection with an EC50 value of 1.2 μM via inhibition of AAK1 kinase (IC50 = 45 nM, Kd = 35 nM). With an IC50 value of 0.6 µM, Erlotinib similarly suppresses HCV infection, but it does so via inhibiting GAK (IC50 = 38 nM, Kd = 21 nM). Both sunitinib and erlotinib are approved kinase drugs for different cancers. Baricitinib, a medication authorised for the treatment of rheumatoid arthritis and myelofibrosis, has recently been recommended as a viable therapeutic option for the treatment of ARDS in COVID19 patients. According to a recent mechanistic study, baricitnib not only reduced ARDS in patients by reducing JAK-1 and JAK-2 signaling, but it also inhibited SARS-CoV-2 entry and intracellular assembly in target cells by inhibiting AAK1 signaling. Baricitinib has been demonstrated to have strong antiviral effectiveness, reducing the SARS-CoV-2 virus load from 30% to 40%, as well as being safe and effective in COVID19 patients, with a significant therapeutic outcome without adverse effects even after two weeks of treatment. Ruxolitinib, a JAK inhibitor used to treat myelofibrosis, also exhibits antiviral effects against HIV and the SARS-CoV-2 virus. Indeed, baricitinib and ruxolitinib are being investigated in clinical trials for the treatment of COVID-19, either alone or in conjunction with other antiviral drugs. Fedratinib is a JAK 2 inhibitor approved for the treatment of myeloproliferative neoplasms that also shows potent antiviral activity against SARS-CoV-2 with an IC50 of 24 nM.
MAPK/ERK pathway inhibitors.
Several studies have found that the MAPK/ERK (mitogen-activated protein kinases/extracellular signal-regulated kinases) signalling is important for viral survival.
The activation of p38 mitogen-activated protein kinase (MAPK) and c-Jun N terminal kinases (JNK1/2) is required for the replication of murine coronavirus (mCoV) and human CoV 229E (HCoV-229E). Although the mechanism by which p38 MAPK contributes to viral replication is unclear, recent investigations have established the role of p38 in the phosphorylation of the first translation factor eIF4E, which is required for viral mRNA translation and subsequent infection of murine CoV (mCoV). Both the influenza A and Ebola viruses employ the p38 MAPK/ERK signaling pathway for entrance and production. The MAPK pathway is important in the early stages of infection in Huh7 human hepatocytes infected with MERS-CoV. Trametinib, an FDA-approved MEK1/2 inhibitor, and Lumitinib, a Phase III MEK1/ERK1/ 2 inhibitor, both demonstrated excellent inhibitory effects (>95%), indicating that medication repurposing might be a feasible option for treating MERS-CoV infection.
Src family of kinases (SFKs) inhibitors. The Src family of kinases (SFKs) has been related to several viruses and has been investigated as a therapeutic target for the treatment of a variety of viral infections. Saracatinib inhibits MERS-CoV with an EC50 of 2.9 µM, as well as hCoV-229E and OC43 with EC50s of 2.4 µM and 5.1 µM, respectively, at early stages of the viral cycle. In an immunofluorescence test, Dasatinib inhibition of Src was strongly associated with the anti-dengue activity. Both Saracatinib and Dasatinib decreased viral infection in Huh-7 and C6/36 cells infected with Dengue fever in a dose-dependent manner. Further research into the mechanism of dengue infection suggests that SRC kinase is required for viral assembly and secretion.
Abl kinase inhibitors. In viruses including the Ebola virus, coxsackievirus, and vaccinia virus, Abl kinases (Abl1 and Abl2) have been involved in various stages of the viral life cycle. Recently, it was shown that Abelson kinase 2 is involved in the fusion of human coronaviruses such as the severe acute respiratory syndrome coronavirus (SARS-CoV-2) and the Middle East respiratory syndrome coronavirus (MERS-CoV). Imatinib and Dasatinib, both authorised Abl inhibitors, were found to be effective antiviral drugs against SARS-CoV and MERS-CoV, with EC50 values of 9.23 µM and 2.1 µM, respectively, according to Dyall et al. Imatinib inhibited viral entrance and replication by inhibiting virions from fusing at the endosomal membrane, according to a mechanistic investigation. In earlier studies, Imatinib has been shown to suppress the Ebola virus, poxvirus, and coxsackievirus. Imatinib, with an IC50 of 130 nM, suppressed SARS-CoV-2 replication, according to Zhao et al. At concentrations of 25 to 3.2 µM, however, it proved toxic.
Nilotinib, a BCR-Abl kinase inhibitor authorised by the FDA (IC50 30 nM), decreased SARS-CoV-1 replication in the micromolar range while inhibiting SARS-CoV-2 replication in the nanomolar range (EC50 80 nM) without causing substantial cytotoxicity. Concerns about Nilotinib's repurposing for COVID-19 arise from its poor pharmacokinetic characteristics. Nilotinib, on the other hand, did not affect MERS-CoV. Bosutinib, originally known as SKI-60, is an FDA-approved BCR-Abl and Src kinase inhibitor for CML. This drug was recently identified as a SARS-CoV-2 inhibitor with an EC50 of 20 nM for the reduction of cytopathic effects in cell-based testing.
Cyclin dependent kinases (CDKs) family inhibitors. Viruses manipulate the expression of cyclin-dependent kinases to control the host cell cycle. CDKs have been associated with HIV, hepatitis B virus (HBV), Zika virus, and herpes simplex virus (HSV). The virus changes the expression and function of host CDKs once it enters, influencing cell cycle progression. Hepatitis B virus increases the activity of the G1-phase kinase CDK4 to enable replication, whereas adenovirus stimulates CDK2 phosphorylation to push progression into late-G1/S-phase.
Many drugs in various stages of clinical studies showed wide spectrum antiviral efficacy against HIV-1, influenza A virus, and Zika virus. Dinaciclib (a CDK1/2/5/9 inhibitor in Phase III), Seliciclib (a CDK2/5 inhibitor in Phase II), Alvocidip (a CDK9 inhibitor in Phase II), and PHA-690509 are among the drugs being studied (CDK2 inhibitor, Phase I). Alvocidib, a flavonoid alkaloid CDK9 kinase inhibitor and commonly known as flavopiridol, showed activity against influenza A virus, in which CDK9 mediated the activity of RdRP. Palbociclib, a CDK4/6 inhibitor that has been approved by the FDA, inhibited HIV-1 and HSV-1 replication in vitro, presumably by blocking cellular protein phosphorylation. A recent study revealed that the SARS-CoV-2 virus can control the cell cycle to boost the viral life cycle. In the Vero E6 and A549-ACE2 cell lines, dinaciclib, an investigational medication that targets CDKs, showed significant anti-SARS-CoV-2 efficacy.Abemaciclib, a selective CDK4/6 inhibitor used to treat metastatic breast cancer, has recently shown specific inhibition of SARS-CoV-2 S (EC50 in Calu3 cell lines). The CPE of SARS-CoV-2 was decreased by 60% using this drug. CDK inhibitors, as part of their antiviral mode of action, stop viral genome replication in host cells. Abemaciclib, on the other hand, reduces coronavirus CPE by preventing cell entrance, suggesting that it might be used as a SARS-CoV-2 entry inhibitor.
Casein kinase 2 (CK2/ CSNK2) inhibitors. CK2 is necessary for the life cycle of a variety of viruses, including the vesicular stomatitis virus (VSV), HIV, HCV, human papillomavirus (HPV), and herpes simplex virus (HSV) (HSV-1). Silmitasertib, a preclinical candidate for treating cholangiocarcinoma and other malignancies by targeting CK2, showed strong antiviral activity (IC50 1.28 M) against SARS-Cov-2, indicating that CK2 plays an important role in the viral life cycle.
PI3K/Akt/mTOR) pathway inhibitors. To support the effective virus replication and escape from the cellular stress responses, different unrelated viruses utilise the phosphatidylinositol 3’-kinase–Akt–mammalian target of rapamycin (PI3K/Akt/mTOR) pathway. HCV, WNV, and IAV all use this route to regulate apoptosis and promote different stages of their life cycles. The activation of this pathway was recently found in MERS-CoV-infected cells. Everolimus, an mTOR inhibitor licensed to treat cancer and prevent transplant rejection, has relatively little anti-MERS-CoV efficacy in vitro. In this infection model, miltefosine, an approved AKT inhibitor, had no impact.
Table 1. FDA approved Kinase inhibitors: Targets, original usage, and repurposing advantages in viral infections.
Drug (brand name) | Kinases as targets | Original use | Repurposing | Benefits |
Gilteritinib (Xospata) | AXL kinase | Acute myeloid leukaemia | SARS-CoV-2 | Antiviral |
Erlotinib (Tarceva) | EGFR tyrosine kinase, GAK, Abl1 | Non-small cell lung cancer (NSCLC) and pancreatic cancer | Dengue and ebolaviruses | Antiviral, anti-fibrotic |
Gefitinib (Iressa) | FTL3/Axl | breast, lung and other cancers | Poxvirus and SARS-CoV-2 | Antiviral |
Sorafenib (Nexavar) | VEGFR, PDGFR, RAF kinases | Renal cell carcinoma, hepatocellular carcinoma, thyroid cancer | SARS-CoV-2 | Antiviral |
Nintedanib (Vargatef) | EGFR | lung cancer and pulmonary fibrosis | SARS-CoV-2 | Antiviral |
Osimertinib (Tagrisso) | EGFR | NSCLC | SARS-CoV-2 | Antiviral, anti-cytokine |
Sunitinib (Sutent ) | AAK1, AXL, GAK, JAK1,KIT | Renal cell carcinoma, gastrointestinal stromal tumor (GIST), | Dengue and ebolaviruses | Antiviral, anti-inflammatory, cytokine suppression, anti-fibrotic |
Baricitinib (Olumiant) | JAK1, JAK2, TYK1 | Rheumatoid arthritis | SARS-CoV-2 | Antiviral, anti-cytokine, anti-inflammatory |
Ruxolitinib (JAKAFI) | JAK1/2 | Myelofibrosis, polycythaemia Vera, and acute graft-versus-host diseases | Ebola, HIV-1,SARS-CoV-2 | Anti-inflammatory, anti-cytokine, anti-fibrotic |
Fedratinib (INREBIC) | JAK2 | Myelofibrosis | SARS-CoV-2 | Anti-inflammatory, anti-cytokine |
Trametinib (Mekinist) | MEK1/2 | Unresectable or metastatic melanoma with the B-Raf-V600E or V600K mutation | MERS-CoV | Antiviral |
Dasatinib (Sprycel) | Abl (Abl-1 & Abl-2) kinase, SFKs (FYN, SRC, YES), CSK | Chronic myeloid leukaemia | SARS-CoV-1,MERS-CoV,dengue virus | Antiviral, anti-inflammatory, cytokine suppression, anti-fibrotic |
Imatinib (Gleevec) | Abl (Abl-1 & Abl-2) kinase | Chronic myeloid leukaemia | SARS-CoV-1,MERS-CoV,SARS-CoV-2, ebolavirus,poxvirus,Coxsackievirus | Antiviral, anti-inflammatory, immunomodulatory/cytokine suppression, anti-fibrotic |
Nilotinib (Tasigna) | Abl (Abl-1 & Abl-2) kinase | Chronic myeloid leukaemia, | SARS-CoV-1, MERS-CoV, | Antiviral, anti-fibrotic |
Bosutinib (SKI-606) | BCR-Abl and Src kinase | chronic myeloid leukemia | SARS-CoV-2 | Antiviral |
Palbociclib (Ibrance) | CDK4/6 | Breast cancer | HSV-1 | Antiviral |
Abemaciclib (Verzenio) | CDK4/6 | Metastatic breast cancer | SARS-CoV-2 | Antiviral |
Evarlimus (RAD-001) | mTOR | cancer and prevent transplant rejection | MERS-CoV | Antiviral |
Conclusions
Kinases are important host cellular targets for the development of antiviral therapy. The use of FDA-approved kinase inhibitors has shown to be highly useful in identifying the role of host kinases in viral infection (see Table 1). However, deleterious effects and pharmacokinetic features must be considered when repurposing kinase inhibitors for the treatment of viral infections.
Acknowledgements
T.P. would like to thank TüCAD2 funded by the Federal Ministry of Education and Research (BMBF) and the Baden-Württemberg Ministry of Science as part of the Excellence Strategy of the German Federal and State Governments.
Keywords: infection, virus, coronavirus, kinase inhibitors, approved drugs, virus entry, replication, life cycle.
References
1. Pillaiyar, T.; Laufer,S.Kinases as potential therapeutic targets for anti-coronaviral therapy. J. Med. Chem. 2021 (doi: 10.1021/acs.jmedchem.1c00335).
2. Raghuvanshi, R.;Bharate, S.B. Recent developments in the use of kinase inhibitors for management of viral infections. J. Med. Chem.2021 (doi: 10.1021/acs.jmedchem.0c01467).
3. García-Cárceles, J.; Caballero, E.; Gil, C.; Martínez, A. Kinase inhibitors as underexplored antiviral agents. J. Med. Chem. 2021 (doi: 10.1021/acs.jmedchem.1c00302).
4. Schor, S.; Einav, S. Repurposing of kinase inhibitors as broad-spectrum antiviral drugs. DNA Cell Biol. 2018, 37, 63-69.
5. Weisberg, E.; Parent, A.; Yang, P. L.; Sattler, M.; Liu, Q.; Liu, Q.; Wang, J.; Meng, C.; Buhrlage, S. J.; Gray, N.; Griffin, J. D. Repurposing of kinase inhibitors for treatment of COVID-19. Pharm. Res. 2020, 37, 167 (doi: 10.1007/s11095-020-02851-7).
6. Attwood, M.M.; Fabbro, D.; Sokolov, A.V.; Knapp, S.; Schiöth,H.B.Trends in kinase drug discovery: targets, indications and inhibitor design. Nat. Rev. Drug Discov.2021 (doi: 10.1038/s41573-021-00303-4)