Lipid-based technologies have been shown to increase bioavailability, and extensive research has been done on the mechanisms by which lipid formulation increases bioavailability. The next stage is to choose the excipient or combination of excipients that is most suited for a robust lipid-based formulation. In this review, we broadly cover the lipids excipients useful in formulation development.
Introduction:
The successful introduction of commercial lipidic formulations of cyclosporine as Sandimmune in 1981, followed by its reformulation as Neoral, has raised interest of the lipid-based drug delivery technology over the past two decades.1 With a greater understanding of the various functions lipids may have in boosting oral bioavailability, interest in Lipid Based Drug Delivery (LBDD) has grown over the past ten years. Additionally, the potential for successful lipid-based formulations has been significantly increased by the emergence of novel excipients with acceptable regulatory and safety profiles along with advancements in formulation technologies. 2
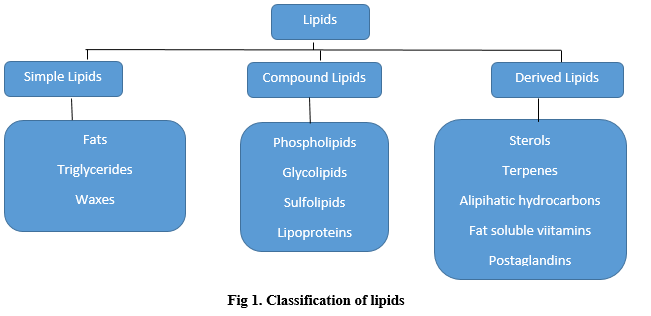
Need of Lipid based drug delivery systems:
Traditional excipients' main functions were binding and adding bulk to the dosage form, facilitating or regulating drug release from the excipient matrix, and facilitating the fabrication of products on high-speed, automated production equipment. But unlike their conventional counterparts, lipid excipients have the capacity to solubilise hydrophobic drugs within the matrix of the dosage form. This frequently leads to an improvement in drug absorption, which is principally mediated by a decrease in the barriers of poor water solubility and slow drug dissolution rate in the gastrointestinal (GI) fluids. Some of these excipients also possess advantageous self-emulsifying abilities, readily forming fine dispersions of lipid-solubilised drug in the GI tract's aqueous contents and creating the optimum circumstances for absorption. 3
Poor water solubility is a major obstacle to efficient drug development for many new therapeutic candidates. It has been predicted that between 70 and 90 percent of all discovery compounds have solubility-limited absorption. Poorly soluble substances typically exhibit inconsistent absorption from the GI tract. Erratic absorption presents safety issues since unpredictable responses could have adverse effects or have no therapeutic effect. This is frequently discovered later in the development process, and in the worst case, when conducting clinical tests, leading to expensive and late project abandonment. The dosage form used to administer the drug is crucial in enhancing absorption for medications that are poorly soluble. Commonly, the formulation is selected by screening a number of standard formulations and, hence, requires compound synthesis. This process is time, labour and cost intensive.4
Interest in lipid-based formulations (LBFs) as a solution for low solubility has developed as a result of the rise in the number of highly lipophilic, poorly water soluble compounds discovered during lead optimisation. Therefore, it is becoming increasingly important for pharmaceutical scientists to develop a suitable formulation that would increase the drug candidate's bioavailability.5 The promise of lipid-based formulation techniques as an alternate method of delivering hydrophobic medications with low oral bioavailability is widely known. They can reduce dosage, eliminate the effects of meals, reduce first pass metabolism by facilitating lymphatic route transfer, and enhance the physical and chemical stability of the drug product, among other benefits. Unlike traditional oral dosage forms like tablets, LBFs often deliver the drug to the stomach in a solubilised state.6 LBFs also maintain the intestinal fluid supersaturated, which increases concentration in the GI tract and makes GI absorption easier. Depending on the molecular characteristics of the drug and the delivery method, LBFs often contain oil, surfactant, co-surfactant, and water-soluble organic solvents in different amounts (i.e. oral, transdermal or injectable formulation). 4
For formulations with a drug suspended or dissolved in lipid excipients, Lipid Based delivery System (LBDDS) is a wide option. One of the industry's most productive and profitable drug development technologies is LBDDS. Lipid vehicles classified based on the chemical structure, polarity of lipids, their characteristics, and the degree of interaction between water and lipids. 7 Use of lipid excipients in the formulation can enhance solubility and dissolution profile of drug candidate, render them in solubilised form suitable for absorption and thus decreases effect of food dependent bioavailability. Selection of lipid excipients affect not only drug solubility in the formulations, but also the solubilisation of drugs in the GI tract during lipid digestion, as well as their absorption and bioavailability.
Importance of Lipid based technology:
The technique is interesting from an academic standpoint on a number of levels, including those that affect solubility, bioperformance, possibility for solidification, etc. Lipid-based formulations are readily available from an industrial standpoint, and there are multitudes of initiatives in the pipeline. Therefore, despite the fact that both academic and industrial perspectives are fascinated, the agendas and demands in the two contexts are distinct. From an industrial standpoint, risks are linked to ambiguity, therefore the more that is understood about a technology, knowledge that may, in theory, be produced in both academics and industry, the better.8
Solubilizing excipients used in commercially available Lipid_based oral formulations:6
Water-insoluble excipients | Triglycerides | Surfactants |
Eg. Beeswax, Oleic acid, Soy fatty acids, Vitamin E, medium chain (C8-C10) Mono- and Diglycerides, Propylene glycol esters of fatty acids | Eg. Corn oil, Olive oil, Peanut oil, Rapseed Oil, Sesame oil, Caprylic /Capric triglycerides derived from coconut oil or palm seed oil | Eg. Glyceryl monooleaste, Cremphor EL, Cremophor RH 40, Cremophor RH 60, Tween 20, Tween 80, Span 20, Labrasol |
Examples of lipids used in different formulations:9
Name of lipid | Type of lipid | Roles in the formulations | Applications |
Magnesium stearate | Simple lipid | In tablets as lubricants, glidants and antiadhesives | Oral drug delivery |
In dry powder inhalations as ‘force control agent’ | Pulmonary drug delivery | ||
Carbowaxes, cholesterol, lecithin, cetyl alcohol, palmityl alcohol | Fatty acids | Emulsion modifiers or emmolients | Topical drug delivery |
Oleic acid, lauric acid, linoleic acid | Fatty acids | Penetration enhancers | Topical drug delivery |
Bees wax | Wax | Emulsifier for emulsions | Used in eye-liners, eye-shadows and mascaras for added hold and shine |
Green tea wax | Wax | Moisturizer, anti-oxidant and emulsifier | Used as colourant for creams and cosmetics |
Intralipid ®, LIPOID S75, Phosphatidylcholine, Distearoyl phosphatidylglycerol | Phospholipids | For formation of lipid bi-layer or vesicle | Novel approach in drug delivery or targeted drug delivery |
Parameters for selection of lipids:10
1. Phase transition temperature
2. Stability
3. Charge
4. Solubility of the drug
5. Source of lipid
Factors affecting choice of excipients for lipid based formulations:11
1. Miscibility
2. Solvent capacity
3. Morphology at room temperature
4. Self-dispersibility and ability to promote self-dispersion of formulation
5. Digestability and fate of digested products
6. Regulatory issues – irritancy, toxicity, knowledge and experience
7. Purity and chemical stability
8. Cost of goods
Examples of lipid based formulation in market:9, 12
Product name | Drug | Dosage form | Company |
Heminevrin® | Clomethiazole | Capsule (soft gelatin) (one-lipid excipient formulations) | AstraZeneka |
Alfarol® | Alfacalcidol | Capsule (soft gelatin), solution and powder (two-lipid excipient formulations) | Chugai Pharmaceuticas |
Norvir® | Ritonavir | Capsule (soft gelatin) (three-lipid excipient formulations) | Abbott Laboratories |
Neoral®/ Sandimmune® | Cyclosporine A | Capsule (soft gelatin) (lipid based microemulsion) | Novartis Pharmaceuticals Corporation |
Sustiva® | Efavirenz | Oral solution | Bristol-Myers Squibb Company |
Cipro TM | Ciprofloxacin | Oral suspension | Bayer Healthcare Pharmaceuticals Inc. |
Agenerase® | Amprenavir | Soft gelatin capsules (SEDDS) | GlaxoSmithKline |
Challenges in development of LBDDS:9
1. Lipids and oil based formulation are susceptible to degradation through lipid peroxidation.
2. Manufacturing process can employ complex and costly instruments and procedures.
3. Not all components are suitable for lipid based formulation due to low solubility or stability issues in the formulation components.
4. The LBF formulator must understand that there will be variations across excipient products, even though they share the same chemical name, as well as slight changes between batches of the same product.
Regulatory perspectives:7, 9
Currently, it is expected that not all excipients are inert, but only a small number could potentially be hazardous. The United States Food & Drug Administration (US-FDA) publishes a list of drugs that are generally recognised as safe (GRAS) prominence in the Code of Federal Regulations (CFR). It also contains the materials listings, which are the so-called inactive ingredient guides for excipients (IIG). The maximum concentration tolerated and a particular route of administration were also included in this IIG guide. The formulator can use the GRAS and IIG lists as reference materials for creating new and generic medicinal products. Detailed safety data may not be necessary for lipids that are already present in conventional formulations. Additionally, the US FDA has provided industry with guidelines for liposomal medicine products. For lipid-based products to be approved, specifics regarding chemistry, manufacturing, human pharmacokinetics, bioavailability, and labelling documentation are needed.
References: