Materials handling – a fundamental decision
All too often the design of pharmaceutical OSD production facilities fails to recognise the impacton, and the opportunities for, materials handling. In this article, Shaun Baker considers a range of different options for materials handling, the benefits and challenges in each case, and the relationship between materials handling and facility design.
Whereas the production processes themselves are easy to define and understand – for example, it is a straightforward task to design a room that fits a tablet press, even if the specific type of machine is undefined – there are many different ways to feed to and from each process.
In the case of a tablet press, the powders or granules can be tipped by hand or vacuum conveyed into the inlet of the machine, so a low height ceiling is acceptable. Alternatively, drums or Intermediate Bulk Containers (IBCs) can be used to feed the tablet press by gravity however a taller room heightwould be a requirement to accommodate this system. A further option is to feedproduct from a mezzanine level or through-floor from a materials handlingfloor above – in this case the height of the compression room can be minimised, but a second production floor level needs to be considered in the facility design.
At the output of the compression process there are more decisions to be made:
Tablets can be received into small tubs, boxes or drums, or collected in larger IBCs within the room, transferred through wall for collection in an adjacent area, or fed through-floor for collection below. It may also be possible to connect the outlet of the tablet press directly to the inlet of a coater or packing machine.
This example only considers one stage of the process – compression – however it illustrates the wide range of materials handling configurations that need to be considered and the impact that these decisions have on the overall facility design.
On the other hand, if you already have an existing facility, then some of these materials handling options will not be available to you. In this case the challenge is to find the optimum materials handling solution that will fit in the space available.
Whether considering a new production facility, or upgrading an existing one, the way that powders, granules, tablets and capsules are moved, stored, fed to and collected from processes has a fundamental impact on how the facility operates: its capacity, flexibility, expandability and cost of production.
Making the right decision at the right stage of the project
Whether you are building a new production facility, or modifying an existing one, the chances are that you will have a good understanding of the process steps that are required at an early stage of the project. This is likely to involve some or all of the following:
Once the process is defined, the next question should be ‘How are the materials (i.e. the powders, granules, tablets and capsules) moved from one process step to another?’ and ‘What is the best way to do this?’. All too often companies fix thefacility layout without fully considering these fundamental questions – the result of which is to limit or compromise on the ideal materials handling solutions.
With materials handling there is not a ‘one size fits all’ solution. Requirements can vary significantly from one project to the next. Factors such as production throughout, number of materials/SKUs, containment requirements, material characteristics, building constraints and available budget all needto be taken into consideration in order to find the right materials handling solution.
What materials handling system is right for my facility?
There are many different ways to move materials through the production process. Three of the most commonly considered materials handling options are:
1. Continuous Processing
2. Handling in drums, tubs, boxes.
3. IBC Systems
1. Continuous Processing
Continuous processing is based on the simple concept of directly connecting the output of one process machine with the inlet of the next.For example, a tablet press (which by its very design is a continuous process) can be directly coupled to a continuous coater, the coater to the packing machine etc.
The benefits of this type of materials handling approach include:
- Materials transfer from process to process without ‘materials handling’
- No connections or disconnections so itcan be particularly suited to the production of high containment products
- Less space required in the facility compared to a batch materials handling system
There is however some limitations with a continuous production approach:
- The production process often involves both batch and continuous processes, and connecting these types of different operations (for example, connecting a batch blender to a tablet press) calls for an intermediate buffer.
- When a number of processes are connected together, the system can only operate at the speed of the slowest process.
- A shutdown of one machine (for maintenance, cleaning or due to a fault) results in the shutdown of the whole process line.
- Continuous processing restricts expandability of a system. For example, if there is a requirement to increase tablet production beyond the capacity of the existing line, then a complete new line is required (possibly requiring a new granulator, blender, compression machine, coater, packing machine etc.)
2. Handling in drums, tubs, boxes etc.
Materials can be collected in small containers at the outlet of one process and manually moved and fed to the inlet of the next process. The powder containers can be tipped into the process by gravity or by use of vacuum transfer, which is also commonin single floor facilities. Manual scooping is a popular way of transferring tablets.
Traditional materials handling approach using small containers
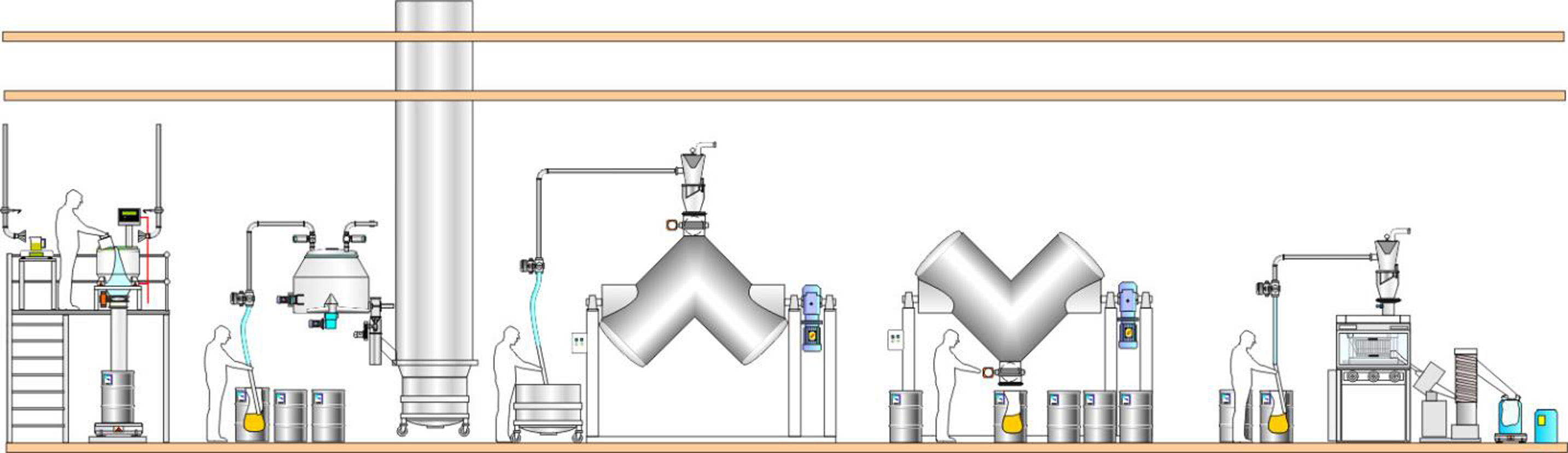
The benefits of this type of materials handling approach include:
- Increased flexibility over Continuous Processing. By moving the containers to where the demand is,it helps balance out the flow of materials through the facility, maintains a reliable supply of material to the packing machines and, in doing so,achieves‘Continuous Manufacturing’.
- Enabling ‘Parallel Processing’ to be carried out - thismeans that processes operate in parallel at their optimum speed, giving the flexibility for any process to connect to any other process. By adopting a Parallel Processing approach, this would allow the output of one blender to feed to many tablet presses for example.
- Keeps the building costs low. The use of small containers and manual operations enables this approach to be used in single floor facilities with small production rooms.
- Low capital cost for material handlingas boxes, drums etc. are cheaper to purchase
However, this traditional approach also has a number of disadvantages:
- This is not a lean approach, as large numbers of containers mean lots of vessel movements, more connections/disconnections, additional cleaning and extra storage.
- Limited production capacity, as the production batch is handled in many small sub-lots and requires lots of manual operations, withtransfers being slow and inefficient.
- Non-contained operation and open transfers increase the risk of contamination and cross-contamination.
- Challenges to inventory control and traceability. As the batch is split into many small sub-lots,this requires manual activities to track each container and results in crowded GMP production areas. As the number of sub-lots increases then the risk for error escalates.
- Labour intensive/reliant, with associated health and safety risks as operators often carry out manual lifting and handling of the containers and materials.
This materials handling approach is normally seen in facilities – or areas of the process – where height and space constraints prevent other options being used.
Sometimes it is possible to upgrade these existing facilities with improved ways of handling powders, granules, tablets and capsules, focussing on bottlenecks in production and considering lean ways to handle materials. However, all too often the constraints of an existing building prevent the use of optimal materials handling solutions.
3. IBC Systems
IBC Systems enable the handling of larger batches in a single container,replacing the many drums, tubs and boxes.
The benefits of IBC Systems include:
- Less containers are required per batch
- Reduced movements of tablet containers
- The number of connections/disconnections are reduced
- Less space is required for storage of containers (when empty and also inter-process)
- Reduced cleaning requirement
How many production floors?
Having decided which materials handling methodology is most suitable, building design is then the next focus.
All over the world, wherever new solid dosage facilities are being built, fundamental choices are being made between building a single-floor or multi-floor plant – and, if multi-floor, then how many floors?
When land is expensive, it makes sense to consider building upwards rather than outwards with a larger footprint. The design of the new facility also needs to balance the future capacity requirements, as well as the requirements on ‘day 1’whilst taking into account the higher investment costs now verses a high modification cost or missed opportunity costs later.
1. Single-floor facility
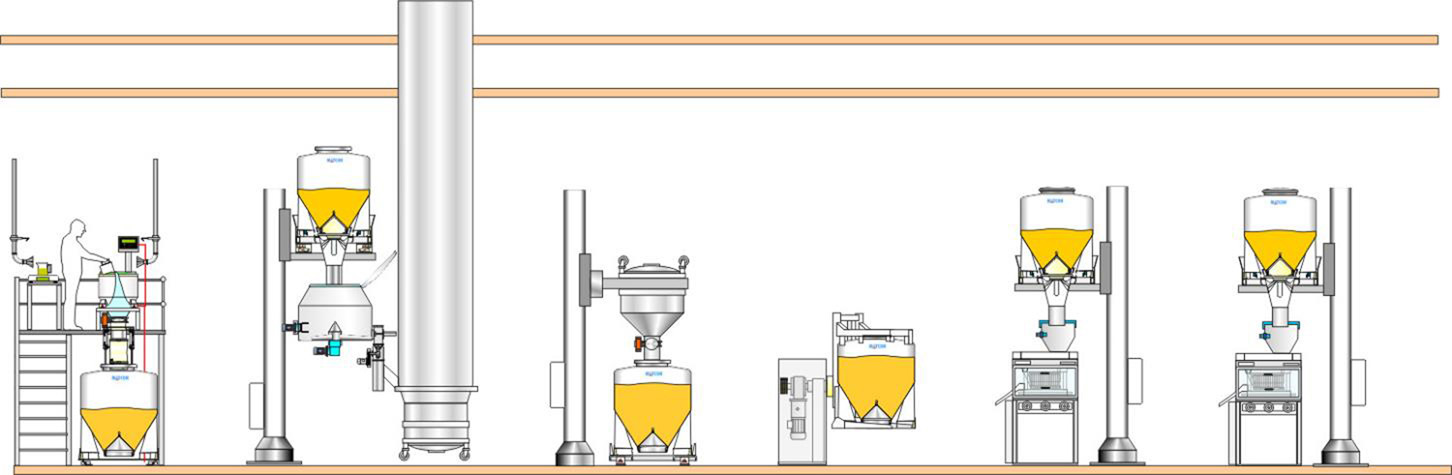

In a single-floor configuration, IBCs can be used to connect one process to another. By matching the volume of the IBC System to the volume of the batch processes, then full batch transfer can be achieved. This means that only one connection/disconnection is required between the IBC System and each process.
Single-floor facilities can have the lowest cost ofconstruction, but they have constraints with regards to the materials handling solutions. To benefit from direct gravity feed, pillar lifts are often used to position IBCs above each process, which means that you must allowfor enough height in the room design to allow the IBCs to be raised up. If the ceiling height is fixed, and too low, you will have to use methodology 2, of boxes and drums, which will require floor space to accommodate the number needed to process a batch.
Frames or mezzanines are alternative ways to achieve gravity feed in single-floor facilities if the building height and layout can accommodate this.
2. Two floor facility
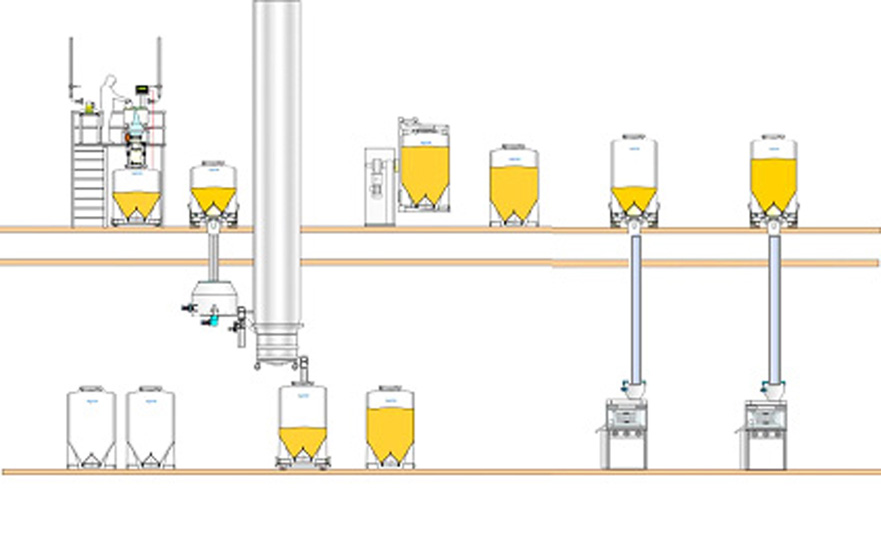
As illustrated by the process flow diagram, a two-floor approach enables the top floor to be dedicated to materials handling and blending, with the tableting and packing processes taking place on the lower floor.In this case, pillar lifts are not required and the height and size of the process rooms can be reduced significantly. The GMP production areas are less crowded and the smaller production rooms are lower cost to construct, maintain and clean.
The top floor can be an open materials handling floor. However, in order to achieve this, it is important to ensure closed connections before, during and after IBC Discharge to provide a dust-tight transfer and avoid the risk of contamination and cross-contamination. Alongside this, the correct solution for gently transferring sensitive materials through-floor also needs to be addressed.
This two-floor arrangement also makes it possible to use larger volume IBCs, enabling production ‘lots’ to be consolidated into larger batch sizes for a more lean approach to manufacturing. As less IBCs are required, the number of IBC movements, connections/disconnections, cleaning requirements and storage space are all reduced.
Multiple floor facility
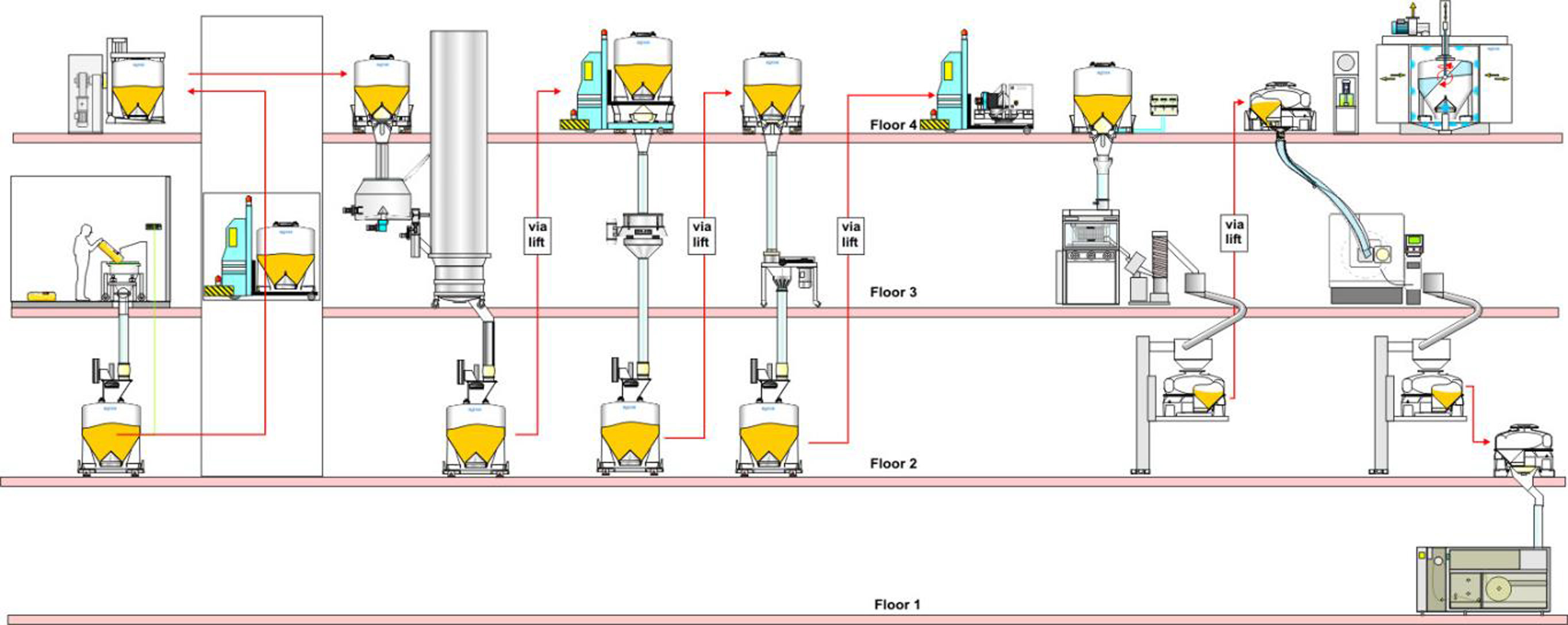
Using more than 2 floors for production, as shown in the diagram, introduces a different type of concept: the ability to completely separate GMP process floors from technical materials handling floors.
The materials handling floors can have a lower area classification as the IBC Discharge Systems and IBC Filling Systems provide separation between the GMP and technical floors. Again, large sized IBCs are ideal for materials handling and can utilise the height of the rooms to maximum effect.
In addition, automation of IBC movements using Automatic Guided Vehicles (AGVs) and automation of cleaning and drying using Clean-In-Place (CIP) equipment, can remove the need for operators on the materials handling floors.
Although this multi-floor arrangement can offer lower cost of manufacture, the investment in the building construction and the high level of automation is only appropriate in certain cases.
Conclusion
Based on my experience working with pharmaceutical companies around the world, I would urge you to consider fourkey points when evaluating your materials handling requirements:
1. There are many different options for materials handling in pharmaceutical OSD production facilities;no ‘one size fits all’ solution and requirements can vary significantly from one application to the next.
2. The way that materials are moved through the facility has an impact on the effectiveness and cost of pharmaceutical manufacture, the specification of the process machines and the design of the facility itself.
3. For new facilities, it is important to review materials handling early in the process, and before the facility design is fixed.
4. Whilst an existing facility design may limit the options available for materials handling, often there are opportunities to significantly improve the way that materials handling is carried out.
The materials handling approach is a fundamental decision for any production facility and it is important that the right choices are made at the right time.
If you would like to discuss the options in further detail, along with how Matcon can add value to your facility, please contact Shaun Baker at [email protected] or visit www.matconibc.com for further information.
Contact:
Matcon Ltd.
Bramley Drive
Vale Park West
Evesham
Worcestershire
WR11 1JH
UK.
Tel: +44 1386 769000
Email: [email protected]
Web: www.matconibc.com