Psoriasis is one of the most prevalent chronic autoimmune inflammatory skin disease affecting 2 to 3 per cent of people worldwide. It is considered as a T-cell mediated autoimmune disorder and is characterised by skin surface inflammation, epidermal proliferation, hyperkeratosis, angiogenesis and anomalouskeratinisation. Current treatment approaches include topical therapy, phototherapy and systemic therapy referred way of treating psoriasis because it allows drugs to be delivered efficiently to target sites of disease, minimising systemic side effects of drugs and ensuring high patient compliance. However, the delivery of anti psoriatic agents via conventional topical formulations like creams, gels, ointments may not be effective due to their poor percutaneous absorption and limited permeability to the deeper layers of the skin. Nanoemulsions are transparent/translucent, isotropic, heterogeneous system of two immiscible liquids consisting of a fine dispersion of drugs in nanodroplets that is stabilisedwith the help of surfactants and co-surfactants. There has been growing interest in exploring its potential in topical delivery for the treatment of various skin diseases such as psoriasis. This review explores the basic principles of the nanoemulsion, formulation aspects and its potential to overcome skin barriers for topical drug delivery.
Psoriasis is an inflammatory, autoimmune disorder of the skin, with a worldwide occurrence of 2-5 per cent. The disease is characterised by inflamed red erythematous plaques supported by silvery scales.1 The appearance of these scaly patches is due to the incomplete cornification and preservation of nuclei in the stratum corneum cells as well as due to the excessive proliferation of epidermis which isthe characteristic feature of Psoriatic skin.2 Histopathological analysis of psoriatic skin reveals hyperplasia of epidermis with significantKeratinocyte differentiation, increased angiogenesis and the presence ofvarious inflammatory infiltrates.3
Depending on the clinical symptoms psoriasis can be classified as guttate psoriasis, inverse psoriasis, plaque psoriasis, erythrodermic psoriasis and pustular psoriasis. The causative factors behind psoriasis are due to both genetic and environmental factors such as streptococcal infection, cutaneous trauma, drugs, alcohol, cigarette smoking, stress and exposure to the UV radiation, which trigger, relapses, but its accurate origin is still not known.4
Treatments available:
Treatment options differ depending on how the formulation is applied or how severe the disease is. Emollients and keratolytic agents were earlier used in psoriasis therapy in order to shed off the skin or hydrate the skin. However, nowadays the focus has shifted to treat the underlying T-cell proliferation. Modern psoriasis treatment regimen includetopical treatments like coal tar, vitamin D, retinoids, topical calcineurin inhibitors and systemic treatments including methotrexate, cyclosporine, acitretin, hydroxyurea. Phototherapy approaches for severe psoriasis have also become more prominent. However, these treatment approaches have severe side effects upon prolonged use. The lack of adequate and safe treatment for psoriasis has created the need to develop new approaches to make the therapy more useful and acceptable.5
Importance of topical therapy in psoriasis and its limitations:
Topical therapy is the preferred choice in the management of psoriasis where high concentrations of drug at the site of action are required for a prolongedperiod. The advantages of topical drug delivery such as good patient compliance, larger surface area for drug delivery, quick termination of therapy and evasion of first pass metabolism have generated immense interest among formulation scientist for the development of topical drug delivery system.6-7
The stratum corneum acts as an effective barrier which limits the effectiveness of the conventional topical formulations such as gels and creams. Hence to overcome this barrier,various approaches including the use of penetration enhancers, electrically assisted methods, nanotechnology-based drug delivery systems arebeing investigated.8-9
Nanoemulsion: A novel drug delivery system for dermal drug delivery
Of the several nanoformulations used to improve topical drug delivery, nanoemulsions are consideredas a potential approach for dermal drug delivery in psoriasis patients. Nanoemulsions are kinetically stable oil-in-water (O/W), water-in-oil (W/O) dispersion of two immiscible liquids stabilised using an appropriate surfactant as shown in figure 1.
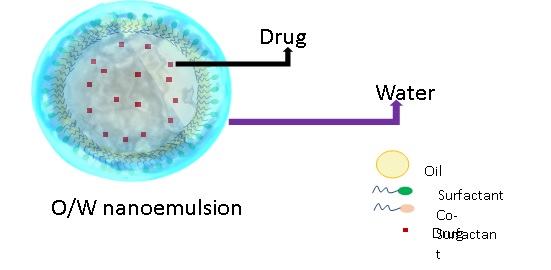
Figure 1: Conceptual Diagram of oil in water Nanoemulsion
Their size varies from 10 to 100 nm. Due to their small droplet, they are clear or transparent distinguishing them from the milky white appearance of coarse emulsions as shown in figure 2.

Figure 2: A Pictorial representation of transparent nanoemulsion v/s coarse Emulsion
The long-term stability of nanoemulsions can be attributed to their small droplet size which overcomes the conventional destabilisation factors such as creaming, sedimentation and coalescence. In the case of nanoemulsions, the Brownian movement is sufficient to overcome any gravity or viscosity induced kinetic instability.10-11
Advantages of nanoemulsion
1. Due to their small droplet size, they have a greater surface area which improves absorption
2. They can be used as an alternative to nanovesicles due to their ease of preparation
3. They are less toxic and irritant
4. It can be incorporated into various topical dosage forms such as creams, gels, and ointments
5. It can be used to solubilise lipophilic drugs as well as hydrophilic drugs
6. Organic solvents need not be used for the preparation of nanoemulsions.12
Types of nanoemulsions
Nanoemulsions can be mainly classified as
• Oil in water nanoemulsions
• Water in oil nanoemulsions
• Bicontinuous nanoemulsions
The interface of all these nanoemulsions become stable only when an appropriate blend of surfactants and co-surfactantsare added.13
Components of nanoemulsion
A typical nanoemulsion contains mainly three components, i.e. oil, Surfactant or/andco-surfactant, water in suitable proportions. The selection of these components and their proportions is made after considering several factors like nature of the drug and excipients,Compatibility issues and Pharmaceutical acceptability. Excipients used should non-irritating to the skin and fall into the GRAS category.
Selection of the Oil Phase
Most of the drugs used in Psoriasis belong the BCS class II and class IV category, hence the oil phase plays a vital role in solubilising these lipophilic drugs. The solubility of the drug in the oil at room temperature is an essential criterion in the selection of the oil phase as higher solubility of the drug in the oil phase at room temperature confirms its solubilisation at body temperature. In most of the cases, GRAS certified, and FDA approved oils such as Sefsol 218, Sefsol 228, Isopropyl myristate are given preference over conventional oils like castor oil, sunflower oil, cottonseed oil and olive oil.14
Selection of surfactant
The selection of a suitable surfactant which can emulsify and solubilise the active ingredient is critical in the formulation of nanoemulsions. Surfactants are also renowned skin permeation enhancers; they help to improve the skin permeation of drugs either alone or in the form of nanoemulsions. There are four categories of surfactant that areused in nanoemulsions for psoriasis treatment. They include cationic, anionic, zwitterionic and non-ionic surfactants. If we take intoconsideration, the nanoemulsions formulated for psoriasis therapy generally oil in water nanoemulsions with non-ionic surfactants having an HLB value between 8-16 are preferred because non-ionic surfactants are comparatively less irritating and then ionic surfactants and have better biological acceptance as well as limited toxicity.
Selection of cosurfactant
The use of a single surfactant may not be sufficient to reduce oil-water interfacial tension to a significant extent. Hence the use of a co-surfactant which is amphiphilic is generally recommended. Cosurfactants act by modifying the curvature and the fluidity of the interfacial film as a result of which they decrease the interfacial tension and increase the entropy of the colloidal system. Nanoemulsions prepared with co-surfactant increase the maximum amount of aqueous phase that can be incorporated in the colloidal system as compared to the surfactant-free system. In most of the cases, C3-C8 alcohols such as propylene glycol, ethylene glycol, Transcutol P, propanol, ethanol are used as co-surfactants.15
Formulation aspects of nanoemulsions
The methods employed to prepare nanoemulsions include high energy and low energy methods. High energy methods involve the input of external energy by using devices such as ultrasonicators, microfluidisers, and high-pressure homogenisers to generatenano-sized droplets and low energy methods include spontaneous emulsification method, solvent evaporation and Phase inversion method. A combination of high and low energy methods are also used in the preparation of nanoemulsions.
High energy homogenisation (HPH)
The method involves passing the oil and water mixture through the narrow orifice of the homogeniser at high pressure resulting in intense turbulence to the mixture. Due to the intense shear and turbulence, the coarse emulsion gets converted into nanoemulsions. The droplet size of these mini emulsions depends on the pressure, on the number of cycles, and temperature of the system. Further, this homogenisation can be done at high temperatures (Hot High-pressure homogenisation) and low temperatures (cold high-pressure homogenisation), cold HPH technique is used for thermolabile drugs. The advantages of this method include industrial scalability, the absence of organic solvents and quick process time.
Microfluidisation
The phenomenon of microfluidisation can also be used to prepare nanoemulsions, where high-pressure displacement pumps are used to prepare nanoemulsions. In this technique, nanoemulsions are formed by the collision between the two immiscible liquids (oil and water) in the interaction chamber or the common impingement area at high pressure and shear. The droplet size of the nanoemulsions formed by this method depends on the pressure employed in operation as well as the number of microchannels that are present in the interaction chamber. The distribution of the droplets in the mini emulsions is much narrower compared to those prepared by other methods. The stability of the emulsions prepared by this method depends on the wetting of the microchannels by the nanoemulsion components. However, this technique is costly and not suitable for large-scale production of nanoemulsions.
Ultrasonication
Kinetically stable nanoemulsions are prepared by this method. The probe sonicatoris brought in contact with the dispersion of liquids containing oil and surfactants which generate ultrasonic waves that is responsible for the generation of the cavitation bubble. These cavitation bubbles collapse and release energy into the system. As a result, small droplets of the dispersed phase with uniform distribution are obtained. The application of high energy may cause decomposition of surfactant molecules. The particle size of the internal phase depends on sonication time, the concentration of the surfactants and power levels. This method is suitable for the production of nanoemulsions on a smaller scale and not for large batches.16-17
Low energy methods
Thermodynamically stable nanoemulsions can be produced by these techniques that have been developed recently. There has been a renewed interest in these methods because of less amount of energy involved in the operation and ease of scalability. These methods alter the HLB balance of the system by using the internal physical properties of the system like temperature and composition.
Phase inversion methods
Phase inversion is achieved by two approaches either by maintaining the composition constant and changing the temperature or by keeping the temperature constant and changing the composition of the systems. Phase in version transition temperature methodis based on the use of temperature-sensitive emulsifiers which changes their affinity to water and oil depending on the temperature of the system. Phase inversion can also be achieved by changing the composition of the system which can be achievedin three ways.
a) Addition of the aqueous phase to the oil-surfactant mixture
b) Addition of oil to the water-surfactant mixture
c) Mixing of all the components of the mixture and emulsification
Phase inversion can also be achieved by the addition of electrolytes, alcohols and surfactants. Due to the change in the HLB of the system phase inversion occurs and changes in spontaneous curvature of the surfactants takes place and nanoemulsions are formed. The limitations of these methods include Ostwald’s ripening, the use of non-ionic surfactants and the need for excellent precision.18-19
Dilution method
One of the simplest methods to prepare nanoemulsions spontaneously is by diluting the microemulsions by a suitable material. Generally, Dilution of O/W microemulsion with water leads to the formation of nanoemulsions. When dilution phenomenon takes diffusion of the surfactant-co surfactant to the aqueous phase takes place as a result of which the droplets lose their thermodynamic stability, and surfactant concentration becomes insufficient to sustain a minimum interfacial droplet tension converting the system to nanoemulsions.20-21
Solvent displacement method
Here the oil phase is dissolved in a suitable organic solvent like methanol or acetone and poured into aqueous phase consisting of surfactants and co-surfactants. Due to the diffusion of the organic solvent spontaneous emulsification occurs and nanoemulsions are formed. The organic solvent used for the preparation of nanoemulsions may be later removed by using the vacuum. The main drawback with this method is thata large amount of solvent is required to produce nanoemulsions.22
Nanoemulsions: How are they formed?
Nanoemulsion formation involves mainly two steps which co-occur,i.e. hydrodynamic breakdown which modulates the suspension of dispersed phases into the dispersion medium and interfacial modification which helps to adsorb the surfactants over the interface. These events in the nanoemulsification take place within a matter of milliseconds. Smaller the droplet size lesser will be the radius of curvature, and higher energy is required for deformation. Hence to produce to small and stable droplets external energy input in the form of agitation, ultrasonic vibration, homogenisation isprovided. The droplets are further stabilised by the use of surfactants which lower the interfacial tension and helps to avoid the excess energy input.23-24
Nanoemulsions: How are they stabilised?
The surfactant-cosurfactant composition, droplet size and zeta potential play a pivotal role in the stability of nanoemulsions. Conventional emulsions have a largerglobule; hence they experience a high gravitational pull, and coalescence of droplets takes place. Whereas in nanoemulsions droplets experience strong intermolecular repulsive forces due to the high degree of surface charge on globules. Hence whenever the droplets come in contact with each other, they experienced strong repulsive forces and moved away after the elastic collision. This phenomenon helps to keep the nanosized droplets in Brownian motion.
The stability of the nanoemulsionis achieved by mainly two kinds of forces,i.e. electrostatic and gravitational force is influenced by droplet size and the electrostatic force is determined by the magnitude of the zeta potential. As a general rule the nanoemulsion containing a single low molecular weight surfactant and having zeta potential above +30mV provide excellentstability, and as the magnitude of zetapotentialincreases, the stability of the formulation further improves.25
When high molecular weight surfactants like tween 80 are used in nanoemulsions, they shift the plane of shear to a greater distance as a result of which there is a decrease in the zeta potential values. However, the nanoemulsionis stabilised by steric hindrance and strong repulsive force between like charges. Even though nanoemulsions are kinetically stable, sedimentation and creamingremain an important stability issue which can overcome by decreasing the globule size, making the dispersion medium viscous and by minimising the differences in density of the dispersed phase and dispersion medium.26-28
Nanoemulsions: How it overcomes the stratum corneum barrier?
Stratum corneumis the principal barrier that must be overcome as far as topical delivery is concerned. The stratum corneum barrier prevents permeation of drugs with a molecular weight above 500 daltons and polar molecules.29-30 For skin delivery, two main approaches are involved,i.e. alteration of the stratum corneum to overcome its barrier properties and distribution of drugs deposited in the stratum corneum to deeper skin layers.
The ability of the nanoemulsions to permeate through the skin can be attributed to their phase structure and particle size. The alteration of the lipid structure, as well as the fluidity of the stratum corneum by the components of the nanoemulsions, is believed to have a significant role in skin permeation. The exact mechanism of dermal penetration by nanoemulsions is not correctly understood some studies have shown that penetration of nanoemulsions to deeper skin layers occurs through intercellular as well as intracellular routes. The ability of nanoemulsions to reduce the stratum corneum barrier by extracting the stratum corneumlipids and denaturing keratinocytes proteins can be another possibility. The presence of sebum in hair follicles may helpin the transfollicular route of penetration. Due to the fluidic microstructure and high skin affinity, nanoemulsions show superior transdermal efficiency compared to rigid nanoparticles such as solid lipid nanoparticles. Another possible mechanism by which the nanoemulsions overcome the biological membrane barrier is by alteration of the cellular arrangement which is reversible and by improving the cell interaction by solubilisation of the lipid barrier or by fusing of the lipid bilayer interface with the cell wall.31-32
Different types of nanoemulsion alters the stratum corneum in different ways, surfactant based nanoemulsions, when used in high concentrations, alter the arrangement of the cells in the stratum corneum and facilitates the paracellular,and transcellular absorption of drugs whereas protein based nanoemulsion undergoes fusion with the cell wall lipid bilayer and releases the drug inside the cells
The surface charge of the nanoemulsion droplets playsa vital role in dermal drug delivery. Hoeller S et al. observed that positively charged nanoemulsion containing phytosphingosine showed better skin delivery of fludrocortisone acetate and flumethasone pivalate through porcine skin when compared to negatively charged nanoemulsions. It is considered that the degree of skin binding is more important in the case of positively charged nanoemulsions since the skin is negatively charged at neutral pH.28
The role of nanoemulsion droplet size in skin permeation is still controversial; it is generally believed that as the droplet size decreases the skin permeability of the drug also improves, and the size of the nanoemulsion droplet determines whether topical delivery or transdermal delivery takes place. Rui Suet al. found that nanoemulsions with droplet size around 50 nm can permeate through the viable epidermis as well as fill the entire hair follicles hence undergo transdermal delivery whereas those with larger size droplets cannot permeate stratum corneum effectively and migrate along the hair follicles.31 Similar observations were reported by Schwarz et al. and Friedman et al.33-34
However recent works have shown that the droplet size of nanoemulsions may not have much effect in skin permeation. Izquierdoet al. compared the skin permeability of tetracaine loaded emulsions and nanoemulsions which had different droplet size but oil and surfactant composition and found that there dermal and transdermal permeation of the drug remained almost the same. These studies vindicated the further need for assessment of the role of nanosized droplets in topical drug delivery.35
Since the psoriatic skin is rough and scaly, it is a significant hindrance in dermal delivery of drugs in psoriasis.Nanoemulsions due to their lipidic nature and small globule size provide good skin retention and drug loading. It is believed that nanoemulsions permeate the scales and rough plaques of psoriatic skin by extraction and swelling of the skin lipids as a result of which enhanced penetration of the nanoemulsion through the pores are achieved.
Several studies have been conducted recently to explorethe utility of nanoemulsions for topical drug delivery in psoriasis, and these studies have proven that nanoemulsions have improved the topical delivery properties compared to conventional topical formulations.
CONCLUSION
Stratum corneum is the primary barrier in the topical delivery of antipsoriatic drugs. Disruption of stratum corneum and targeting to the deeper layers of skin are not possible with conventional topical dosage forms like gels and creams. Hence nanotechnology-based drug delivery systems like nanoemulsions are employed to overcome these limitations. Nanoemulsions are kinetically stable systems that can effectively overcome the stratum corneum on account of its surface charge, lipid nature and droplet size. Aright combination of the suitablemethod of preparation, oil and surfactant-co surfactant mixture can enhance effective dermal delivery of antipsoriaticdrugs thus improving the psoriasis therapy.
CONFLICT OF INTEREST
The authors declare that there is no conflict of interest.
References
1. Avasatthi V, Pawar H, Dora CP, Bansod P, Gill MS, Suresh S. A novel nanogel formulation of methotrexate for topical treatment of psoriasis: optimization, in vitro and in vivo evaluation.Pharm Dev Technol. 2016;21(5):554-62.
2. Thappa D, Malathi M. Topical therapy of psoriasis: Where do we stand? J Postgrad Med. 2017;63(4):210-12.
3. De Rosa G, Mignogna C. The histopathology of psoriasis. Reumatismo. 2007;59(1s):46-8.
4. Naldi L, Gambini D. The clinical spectrum of psoriasis. Clin Dermatol. 2007;25(6):510-8.
5. Raut AS, Prabhu RH, Patravale VB. Psoriasis clinical implications and treatment: a review. Crit Rev Ther Drug Carrier Syst. 2013;30(3):183-16.
6. Patel V, Sharma OP, Mehta T. Nanocrystal: a novel approach to overcome skin barriers for improved topical drug delivery. Expert Opin Drug Deliv. 2018;15(4):351-68.
7. Schäfer-Korting M, Mehnert W, Korting H-C. Lipid nanoparticles for improved topical application of drugs for skin diseases. Adv Drug Deliv Rev. 2007;59(6):427-43.
8. Barry BW. Novel mechanisms and devices to enable successful transdermal drug delivery.Eur J Pharm Sci. 2001;14(2):101-14.
9. Barry BW. Breaching the skin's barrier to drugs. Nat Biotechnol. 2004;22(2):165-7.
10. Solans C, Izquierdo P, Nolla J, Azemar N, Garcia-Celma M. Nano-emulsions. Curr. Opin. Colloid Interface Sci. 2005;10(3-4):102-10.
11. Singh Y, Meher JG, Raval K, Khan FA, Chaurasia M, Jain NK, et al. Nanoemulsion: Concepts, development and applications in drug delivery. J Control Release. 2017;252:28-49.
12. Jaiswal M, Dudhe R, Sharma P. Nanoemulsion: an advanced mode of drug delivery system. 3 Biotech. 2015;5(2):123-7.
13. Li J, Shi Y, Ren Y, Cong Z, Wu G, Chen N, et al. Development and evaluation of self–nanoemulsifying drug delivery system of rhubarb anthraquinones. J Drug Deliv Sci Technol. 2017;39:283-95.
14. Rai VK, Mishra N, Yadav KS, Yadav NP. Nanoemulsion as pharmaceutical carrier for dermal and transdermal drug delivery: Formulation development, stability issues, basic considerations and applications. J Control Release. 2018;270:203-25.
15. Fasolin L, Santana R, Cunha R. Microemulsions and liquid crystalline formulated with triacylglycerols: Effect of ethanol and oil unsaturation.Colloids Surf A Physicochem Eng Asp. 2012;415:31-40.
16. Solans C, Solé I. Nano-emulsions: formation by low-energy methods. Curr. Opin. Colloid Interface Sci. 2012;17(5):246-54.
17. Maali A, Mosavian MH. Preparation and application of nanoemulsions in the last decade (2000–2010). J Dispers Sci Technol. 2013;34(1):92-105.
18. Gutiérrez J, González C, Maestro A, Solè I, Pey C, Nolla J. Nano-emulsions: New applications and optimization of their preparation. Curr Opin Colloid Interface Sci. 2008;13(4):245-51.
19. Izquierdo P, Esquena J, Tadros TF, Dederen C, Garcia M, Azemar N, et al. Formation and stability of nano-emulsions prepared using the phase inversion temperature method. Langmuir. 2002;18(1):26-30.
20. Wang L, Mutch KJ, Eastoe J, Heenan RK, Dong J. Nanoemulsions prepared by a two-step low-energy process. Langmuir. 2008;24(12):6092-9.
21. Solè I, Solans C, Maestro A, González C, Gutiérrez J. Study of nano-emulsion formation by dilution of microemulsions.J Colloid Interface Sci. 2012;376(1):133-9.
22. Trimaille T, Chaix C, Delair T, Pichot C, Teixeira H, Dubernet C, et al. Interfacial deposition of functionalized copolymers onto nanoemulsions produced by the solvent displacement method. Colloid Polym Sci. 2001;279(8):784-92.
23. Tadros T, Izquierdo P, Esquena J, Solans C. Formation and stability of nano-emulsions. Adv Colloid Interface Sci. 2004;108:303-18.
24. Gupta A, Eral HB, Hatton TA, Doyle PS. Nanoemulsions: formation, properties and applications. Soft matter. 2016;12(11):2826-41.
25. Kong M, Park HJ. Stability investigation of hyaluronic acid based nanoemulsion and its potential as transdermal carrier. Carbohydr Polym. 2011;83(3):1303-10.
26. Koroleva MY, Yurtov EVe. Nanoemulsions: the properties, methods of preparation and promising applications. Russ Chem Rev. 2012;81(1):21-43.
27. Liu W, Sun D, Li C, Liu Q, Xu J. Formation and stability of paraffin oil-in-water nano-emulsions prepared by the emulsion inversion point method. J Colloid Interface Sci. 2006;303(2):557-63.
28. Hoeller S, Sperger A, Valenta C. Lecithin based nanoemulsions: A comparative study of the influence of non-ionic surfactants and the cationic phytosphingosine on physicochemical behaviour and skin permeation. Int J Pharm.. 2009;370(1-2):181-6.
29. Nair A, Jacob S, Al-Dhubiab B, Attimarad M, Harsha S. Basic considerations in the dermatokinetics of topical formulations. Braz J Pharm Sci. 2013;49(3):423-34.
30. Bos JD, Meinardi MM. The 500 Dalton rule for the skin penetration of chemical compounds and drugs. Exp Dermatol. 2000;9(3):165-9.
31. Su R, Fan W, Yu Q, Dong X, Qi J, Zhu Q, et al. Size-dependent penetration of nanoemulsions into epidermis and hair follicles: implications for transdermal delivery and immunization. Oncotarget. 2017;8(24):38214-26.
32.Shakeel F, Baboota S, Ahuja A, Ali J, Shafiq S. Skin permeation mechanism of aceclofenac using novel nanoemulsion formulation. Pharmazie.. 2008;63(8):580-4.
33. Friedman DI, Schwarz JS, Weisspapir M. Submicron emulsion vehicle for enhanced transdermal delivery of steroidal and nonsteroidal antiinflammatory drugs. J Pharm Sci. 1995;84(3):324-9.
34. Schwarz JS, Weisspapir MR, Friedman DI. Enhanced transdermal delivery of diazepam by submicron emulsion (SME) creams. Pharm Res. 1995;12(5):687-92.
35. Izquierdo P, Wiechers J, Escribano E, Garcia-Celma M, Tadros TF, Esquena J, et al. A study on the influence of emulsion droplet size on the skin penetration of tetracaine. Skin Pharmacol Physiol.2007;20(5):263-70.