Age-related Macular Degeneration (AMD) is the third leading cause of worldwide blindness that causes permanent central vision impairment in older people. Currently there are limited diagnostic and therapeutic strategies available to manage AMD. Various engineered nanoformulations, composed of polymers, lipids, proteins, inorganic materials, have been significantly investigated for the management of AMD. This article highlights the progress and future perspectives of nanodiagnostics and nanotherapeutics.
Introduction
AMD accounts for the progressive deterioration of the Retinal Pigment Epithelium (RPE) function and loss of specialised neuroepithelial cells (photoreceptors) in the macula, central part of the retina at the back of the eye, responsible for central vision and therefore result in blurred and distorted vision [1]. Further, it can cause irreversible blindness in many patients. Early-stage AMD is characterised by the occurrence of drusen and abnormalities in RPE. Late-stage AMD has been clinically categorised as wet and dry AMD. Dry AMD (also known as geographic atrophy (GA), non-neovascular or non-exudative) refers to the clumped lipids and proteins ‘drusens’accumulating beneath the macula that progressively thins the retina structure and causes thickening of the Bruch’s membrane resulting in vision impairment. Wet AMD or Choroidal Neovascularisation (CNV) as is a chronic and progressive formation of new blood vessel (neovascularisations or angiogenesis) within the retina accompanied by overexpression of Vascular Endothelial Growth Factor (VEGF) and results in the degeneration of the central retina.
The pathogenesis of nAMD is primarily attributed to oxidative stress due to Reactive Oxygen Species (ROS), accumulation of retinal toxins, parainflammation and complement system dysfunction. VEGF or Vascular Permeability Factor (VPF) is an endothelial cell-specific mitogen that stimulates the angiogenesis (new blood vessels formation) by inducing proliferation, migration, and permeability of endothelial cells. VEGF plays a central role in AMD manifestation. It has been detected in high concentrations in CNV membranes and aqueous humor of AMD patients as compared to healthy volunteers, thereby implicating the link between VEGF gene expression and the progression of CNV. Therefore, the role of VEGF is central to design of treatment modalities for AMD.
Therapeutic strategies for AMD
Despite the growing scientific and technological advancements made in the field of ophthalmic therapeutics, there is no satisfactory reliable therapy available today. The composite network of the ocular tissues acts as potential barriers and prevents topical drugs to diffuse in the retina. The anatomical (static) barriers and physiological (dynamic) barriers compositely cause reduced bioavailability of topical drugs at the retinal surface. The decreased bioavailability (<5%) of the drug necessitates frequent administrations restricting its feasibility as an AMD treatment. At present palliative treatments include intravitreous drugs, subconjuctivaland subretinal injection, topical administration, laser therapy and Dynamic Phototherapy (PDT).
Since PDT specifically targets CNV, it should provide an optimum treatment modality with minimum damage to the retinal surface. PDT as an approach can significantly cause vision loss and severe side effects including deterioration of RPE and photoreceptors. To overcome the cons of the PDT modality, anti- VEGF drugs are increasingly espoused. However, the major drawback of IVT of anti – VEGF drugs is its invasive nature that is painful, limited uptake, and ocular penetration and intraocular bleeding accompanied with patient discomfort. Furthermore, injections inherently specify sterility to prevent pathogenic infestations that otherwise cause endophthalmitis, this further may complicate requiring vitrectomy. It also necessitates injection administration for a long period due to its recurrent nature and post-injection complications involving locally affected tissues due to RPE tear, increased intraocular pressure, intraocular inflammation, vitreous hemorrhage and detachment of retina.
To overcome several limitations current available therapeutic strategies explore specific nanoformulations. The nanoformulations today include drug delivery systems involving drug complexes like cyclodextrin-drug conjugate, lipid NPs, and inorganic NPs. These cyclodextrin-drug conjugates and NPs with lipid inclusion poses the unfortunate short half-life and short drug release profiles thereby requiring frequent drug administration. The present disadvantage of intravitreal administration of NPs is the generation of concerns regarding the risk of post-injection. This necessitates the need to develop non-invasive therapies that efficaciously cross the physiological and anatomical ocular barrier but also provide the encapsulated drug for longer durations [2], [3].
Nanotechnology-based platforms currently deliver the drugs safely and effectively to the target site of the eye by reducing the frequency of IVT injection as well as post-injection patient complications or adverse reactions. The nanoparticulates strategies for the diagnosis and treatment of AMD are presented and discussed in the next sections.
Diagnostic strategies of AMD
Early clinical diagnosis is a critical aspect of management and prevention of late-stage AMD and long-term complications. Most of the time, delayed diagnosis or inaccurate examination can cause the treatment to prolong and lead to other complications requiring combination treatment. The diagnostic approaches today in prevalence are based on imaging techniques that provide diagnostic features ranging from older techniques based on film imaging to newer digital techniques. Diagnostic modalities for AMD shown in Fig. 2, include autofluorescence photography, dilated eye examination, fundoscopy or ophthalmoscopy, visual acuity eye testing, fundus photography, fluorescein angiography, tonometry, Optical Coherence Tomography (OCT), amsler grids, noise-field perimetry, Macular Mapping Test (MMT), Preferential Hyperacuity Perimeter (PHP) and artificial intelligence. Further technological advancements will yield multifaceted versions of existing systems which function at tremendous speed to furnish images with enhanced resolutions. This will increase the capacity of the diagnosis for its application [4].
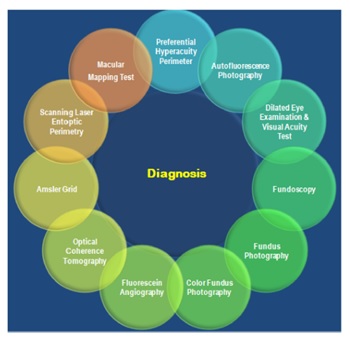
Figure 1. Diagnostic modalities for AMD [1]
Nanodiagnostics and Nanotherapeutics for nAMD
The past few decades have seen the revolutionary intervention of nanotechnology that offers immense opportunity to create novel ocular delivery systems for safe and effective delivery of anti-VEGF agents for treating AMD. NPs are sub nanosized (10–1000 nm) colloidal particles that include polymer, lipid, inorganic, and hybrid (multi-functional) based systems that can reduce the toxicity of drugs by delivering them to the retinal targeted site with appropriate concentration. Nanoparticulate delivery systems provide benefits that include improved bioavailability, enhanced drug pharmacokinetics, and pharmacodynamics, control release, accumulation in areas of increased vascular permeation, non-specific toxicity, and lower immunogenicity. These advantages overcome the major obstacles faced in ocular drug administration.
Nanopharmaceuticals loaded with drugs can improve drug efficacy with the implementation of bio-recognition. Moreover, particle size, shape, surface charge, andother physicochemical properties of NPs can also influence penetration through ocular tissues compared to conventional delivery systems. Polymer-based nanoparticles are made up of non-toxic, non-immunogenic, biodegradable, and biocompatible polymers such as Poly (D, Llactide- co-glycolide) (PLGA), chitosan, Polyethylene Glycol (PEG), albumin and Hyaluronic Acid (HA). Bio- or mucoadhesive (for example, chitosan) polymer enhances the bioavailability of drugs by increasing the time of residence within the mucosal layer. Lipid-based NPs can be used to generate liposomes, Solid Lipid NP (SLN), nanostructured lipid carriers (NLC) that consist of a lipid encapsulating cargo molecules. Metal (gold, silver, iron oxide) NPs are used to invade and reach deep layers of the retina and choroidal space via IVT injection.
With the advent of improved instrumentation and characterisation techniques, advances have been introduced at an exponential rate in the field of nanotechnology and applied molecular biology with great potential for medical applications. Although intraocular or periocular administration may achieve high therapeutic concentrations at the target site with topical or systemic drugs, they pose major issues. Themajor drawback of topically administered drugs and Intravitreal Injections (IVT) of anti-VEGF based therapeutics is decreased bioavailability due to small structural space, tear drainage and metabolic degradation of drugs, barriers in the ocular anatomy and the chance of contracting infection upon unsterilised injection [5].
NPs are formed of multiple materials with multifactorial groups on its large surface area to reach specific sites of the eye. Interaction of NPs with ocular mucus layer or cells prolongs the residence time of anti- VEGF drugs loaded NPs. These NPs improve the bioavailability of drugs at the extraocular tissues by reducing the drainage of drugs and enhancing the penetration of drugs through the ocular surface tissues to periocular tissues. These NPs can be synthesised with polymers, lipids, an inorganic material, or a combination of various sizes and conformations with modulation to incorporate biodegradable properties. Fig. 2 shows schematic illustration of different types of nanoparticles used for AMD therapy.
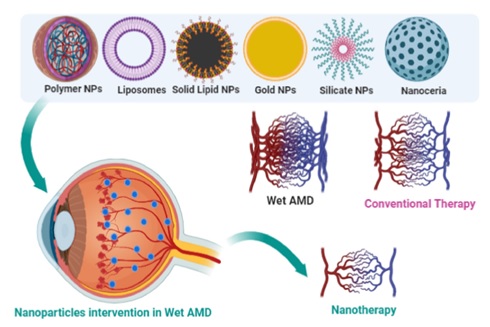
Fig. 2. Schematic illustration of different types of nanoparticles used for AMD therapy. [1]
Concluding Remarks and Future perspectives
In the last few decades, though the manifestation of nAMD associated irreversible blindness in the elderly can be prevented in a larger population of patients, there are still many unresolved challenges with frequent IVT injections of anti-VEGF agents. At present, the development of safe and effective therapeutics is needed on an urgent basis. Moreover, a deep understanding of the disease pathogenesis and the static or dynamic ocular drug delivery barriers can help to address the various drug bioavailability, safety, efficacy, and drug administration related issues for improved therapy. Bioavailability of drugs can be enhanced by either ‘prolonging retention time’using mucoadhesive formulations, positively charged formulations, thermosensitive formulations, controlled release formulations or ‘enhancing corneal permeability’using cell-penetrating peptides. Injectable nanospheres could be a promising alternative for ocular implants. Thus, diagnostic and therapeutic tools include nanotechnology, photodynamic therapy, stem cell therapy, gene therapy, artificial intelligence, and 3D printing strategies that are likely to have a major impact on the diagnosis, treatment, and management of nAMD in the future.
References
[1] Sarkar, Aira, and SathishDyawanapelly. "Nanodiagnostics and Nanotherapeutics for age-related macular degeneration." J. Con Rel. 329 (2020). Volume, 1262-1282
[2] W.L. Wong, X. Su, X. Li, C.M.G. Cheung, R. Klein, C.-Y. Cheng, T.Y. Wong, Global prevalence of age-related macular degeneration and disease burden projection for 2020 and 2040: a systematic review and meta-analysis, Lancet Glob. Health. 2 (2014) 106–116.
[3] P.M. Hughes, O. Olejnik, J.-E. Chang-Lin, C.G. Wilson, Topical and systemic drug delivery to the posterior segments, Adv. Drug Deliv. Rev. 57 (2005) 2010–2032.
[4] S.J. Kim, The role of imaging in the diagnosis and management of uveitis, Expert Rev. Ophthalmol. 5 (2010) 699–713.
[5] J.G. Christie, U.B. Kompella, Ophthalmic light sensitive nanocarrier systems, Drug Discov. Today 13 (2008) 124–134.