Chemotherapeutics drugs play a pivotal role in the treatment of cancer, but they have a negative impact on therapeutic outcomes. So, co-delivery strategies for administration of siRNA and chemotherapeutic drugs is gaining potential importance for treatment of many cancers. The ideal combination of chemotherapeutic drugs with siRNA is very crucial for producing the desirable anticancer effects.
Introduction
Cancer is a dynamic cellular process in which complex inter-play of cellular interactions and genetic alterations characterised by multiple gene mutations occur. The complexity of signaling pathways makes treatment of cancer difficult due to altered cellular mechanisms which cause cell death [1]. This calls for treatment modalities which can target the molecular mechanisms involved in cancer patho- physiology. Therefore, as a supplement to the existing therapy, combinatorial approaches are being implemented in the treatment of different types of cancers wherein along with chemotherapeutic agents Small interfering RNA (siRNA) are being administered [2].In this line, the co-administration of chemotherapeutic drugs and small interfering RNA (siRNA) can act as a potential tool for the treatment of cancer and has attracted the attention of oncologists and researchers throughout the world.
The communication highlights and emphasizes on the various research outcomes which can be potentially used for the treatment of cancer.
RNA interference (RNAi) technology
RNA interference (RNAi) technology as shown in Table 1 is being investigated as a possible strategy for developing highly targeted RNA based gene-silencing in cancer management. RNA interference (RNAi) inhibit expression of genes by binding to target messenger RNA (mRNA) and disrupt translation once they reach the cytoplasmic RNA-induced silencing complex (RISC) as shown in figure 1[3].
| RNAi approaches | Description | Target mechanism |
siRNA (Small interfering RNA) | Double-stranded RNA (dsRNA) oligonucleotides (21–25 base pair) that are either generated artificially or are endogenous dsRNA products. | siRNA is bound by RNAinduced silencing complex (RISC) and unwound it into single-stranded strands that bind to the complementary mRNA sequence. The pairing causes cleavage at the target sequence, and the cleaved mRNA is degraded and prevented from being translation[4]. |
shRNA (Short hairpin RNA) | shRNA is made up of a 19–20 base pair RNA sequence with a short hairpin loop of 4–11 nucleic acid. | The shRNA-plasmid enters the cell, integrates with the host nuclear DNA, and generates pre shRNAs that are transferred into the cytoplasm, where the shRNA cleaves the target mRNA with the help of the dicer complex[5]. |
| lncRNA (Long noncoding RNAs) | lncRNA are made up of over 200 nucleotides. However, they lack the ability to code for proteins. | These RNAs may be involved in chromatin remodeling, transcription regulation, and RNA processing in the nucleus, but in the cytoplasm they usually perform their functions by interacting with mRNAs and proteins[6], [7]. |
miRNA (Micro RNA) | miRNAs are single-stranded, non-coding RNA oligonucleotides (20–25 base pairs). Multiple siRNAs can be created from a single miRNA transcript. | They target mRNAs with basepair recognition and initiate mRNA degradation, which reduces the levels of the respective protein[8]. |
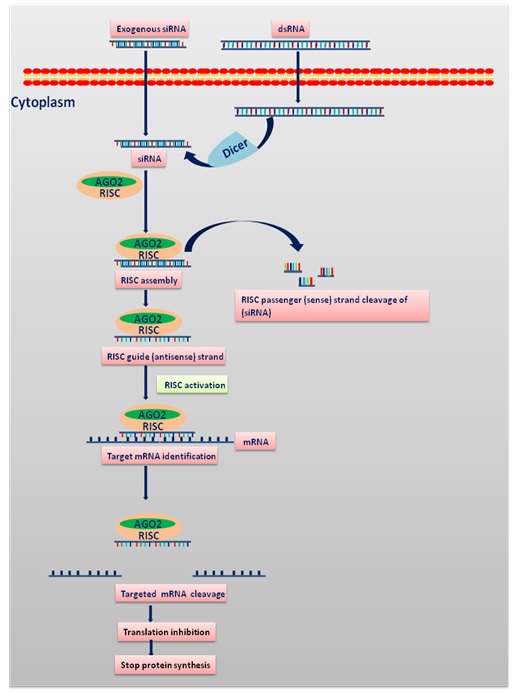
Figure.1 Process of siRNA mediated RNA interference: The process initiates with cleaving of long double-stranded RNA (dsRNA) into siRNA or introducing exogenous siRNA into the cytoplasm with the Dicer enzyme complex. This is followed by binding of siRNAs to Argonaute 2 (AGO2) and the RNA-induced silencing complex (RISC).If complementarity of sequence is established between the RNA duplex packed onto RISC, AGO2 cleaves the passenger (sense) strand, resulting in active RISC containing the guide (antisense) strand. The siRNA guide strand helps to block the protein synthesis by binding to the target sites for direct mRNA cleavage (brought out via catalytic domain of AGO2)[9].
The rationale for selecting chemotherapeutic drug-siRNA pair for treatment of cancer
Combinatorial pairing of siRNAs and chemotherapeutic drug is very critical to the targeting of cellular pathways, multiple effects such as combination of anti-angiogenesis effect, or anti-proliferation effect, or reversal of MDR, or inhibition of metastasis (invasion) can be achieved. The suitable pair selection can thus provide many-fold increase in antitumor activity with the reduction of the dose [10]. The advantages of co-administration of siRNA with chemotherapeutic drugs which target various mechanisms for management of cancer have been demonstrated in figure 2.
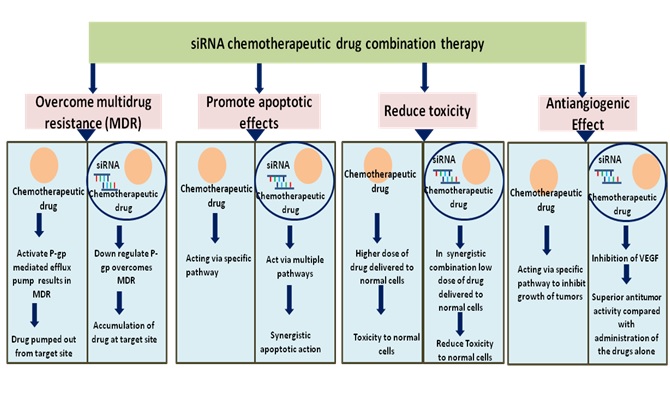
Figure.2 Advantages of combination therapy of siRNA with chemotherapeutic drug for safe and targeted treatment of cancer.
Significance for chemotherapeutic drugs and siRNA combination mechanisms
The success of the combination therapy along depends on the identification of the suitable gene target. The following genes have been targeted using this combinatorial therapy: (1) genes which play a role in cancer drug resistance (e.g., a gene encoding P-gp) (2) genes responsible for survival of tumors (e.g., gene encoding Bcl-2) (3) genes expressed selectively in cancerous tissues (e.g., gene encoding VEGF) [11]. Targeting of the distinct cellular signaling pathways, enhances the genetic hurdles for cancerous cells mutations resulting in retardation of the tumor adaptation system thereby improving therapeutic efficacy
Carrier systems for co-delivery of chemotherapeutic agents and siRNA
For the targeted and efficient delivery of nucleic –acid based drugs, suitable drug carriers are also required, as nucleic acids, including siRNAs, are water-soluble and negatively charged so it is difficult to deliver them through the cell membrane. For being delivered to the site of action for exerting their effect, they must escape in the intact form from endosomes and lysosomes. Furthermore, they must be protected against degradation by the endogenous enzymes and media along with the formulation excipients [12]. The extracellular barriers to RNAi-mediated siRNA delivery have been described in figure 3 and these barriers are being exploited by the researchers as potential delivery sites for the nucleic-acid based molecules. Herein, two types of delivery techniques: viral vectors-based and non-viral-based delivery techniques have been discussed.
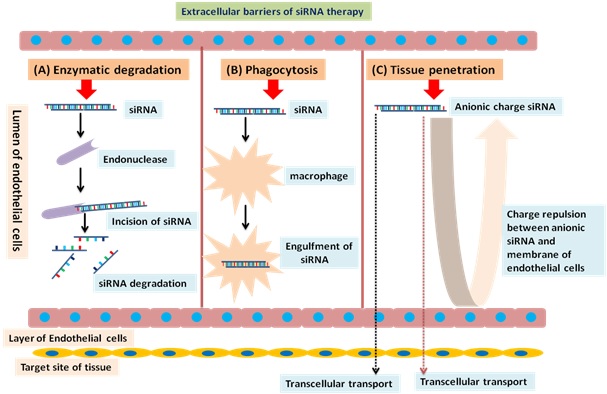
Figure 3. Extracellular barriers to RNAi mediated siRNA therapy (A) Enzymatic degradation: Endonuclease degrades siRNA in blood circulation (B) Phagocytosis: The phagocyte cells (macrophages) removes siRNA from blood circulation via clearance through liver, lungs, and spleen (C) Tissue penetration: Repulsive interactions (due to anionic charge) between siRNA and plasma membrane of endothelial cells prevent internalisation of siRNA into cells, and are transported only by transcellular and paracellular transport mechanisms.
Nanocarrier chemotherapeutics-siRNA co-delivery system
Nanoparticles have the capacity to co-encapsulate chemotherapeutic drugs and siRNA and, they have been used to produce synergistic anticancer effect as shown in figure 4[13]. Various endocytotic mechanisms are available for the delivery of nanocarriers, like clathrin-based endocytosis, caveolae-based endocytosis, macropinocytosis, and additional clathrin and caveolae-independent endocytosis processes. Degradation of nanocarriersin the endosomes occurs by the lysosome (pH 4.8), facilitated by the acidic pH and degradative enzymes in the breakdown of these carriers and its payloads (e.g. siRNA). siRNAs need to escape the endosome during the loading period otherwise it may lead to lysosomal breakdown. Photochemical internalisation mechanism helps in endosomal escape of nanocarriers, as a result of the proton sponge effect, endosome membrane fusion, pore formation, and flip flop mechanism, among other strategies.[14].
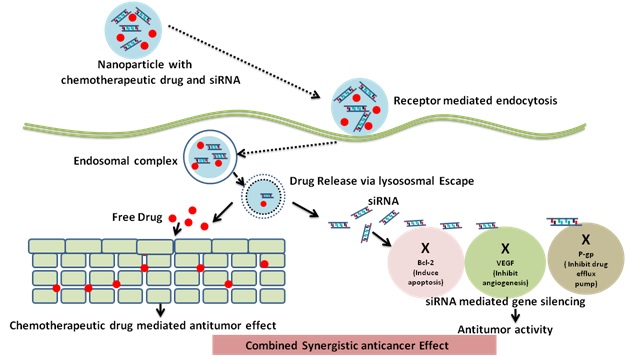
Figure 4.Schematic diagram of nanocarrier chemotherapeutics-siRNA co-delivery system for synergistic anticancer effect.
Table 1. Various nanocarrier systems for co-delivery of chemotherapeutic drugs and siRNA for tumor regression
| Nanocarrier | Chemotherapeutic drug | siRNA | Targeted gene | Targeted cell line | References |
| Polyethylenimine (PEI)-functionalised graphene oxide (PEI-GO) | Doxorubicin | Bcl-2 siRNA | Bcl-2 | HeLa cells | [15] |
| Mesoporous silica nanoparticle (MSNP) | Doxorubicin | Pgp-siRNA | P-glycoprotein (Pgp) | Breast cancer cell line MCF-7/MDR cells | [16] |
| Trimethyl chitosan nanoparticles | Doxorubicin | HMGA-2 siRNA | HMGA-2, vimentin, and MMP9 | Breast cancer cell line (MDA-MB-231) | [17] |
| Nanostructured lipid carriers(NLCs) | Gefitinib and Paclitaxel | EGFR siRNA | EGFR | Human lung cancer A549, PC-9, PC-9GR, and H-1975 cell | [18] |
| Cationic solid lipid nanoparticles | Paclitaxel | MCL1 siRNA | MCL1 | KB cells | [19] |
| Cationic Liposome | Adriamycin | siRNA RRM2 | RRM2 , EGFR antibody | HCC cells | [20] |
| (FA) -conjugated polyamidoaminedendrimer | Cis-diamine platinum (CDDP) | HuR siRNA | HuR, Folate receptor-α (FRA) | H1299 lung cancer cells | [21] |
Conclusion
The shortfalls of the current cancer chemotherapy like sever adverse effects, drug resistance, and high dose of the drugs, RNA interference (RNAi) therapy is being explored as a promising targeted treatment strategy. Use of siRNA can be adopted as a potential method to down regulate the genes responsible for drug resistance and chemotherapeutic ineffectiveness. Co-delivery helps to increase the therapeutic efficacy by providing synergistic or additive effects thereby multi-drug resistance and the side effects. The combinatorial therapy also helps to target multiple pathways and regulatory proteins related to cancer cell growth, metastatic spread, and drug resistance. Despite considerable progress over the past few decades, there are still a number of obstacles to be overcome before siRNA and drugs can be successfully delivered together, so that it can be clinically translated.
References:
[1] J. Li, Y. Wang, Y. Zhu, and D. Oupický, “Recent advances in delivery of drug-nucleic acid combinations for cancer treatment,” J. Control. Release, vol. 172, no. 2, pp. 589–600, 2013, doi: 10.1016/j.jconrel.2013.04.010.
[2] A. Judge and I. MacLachlan, “Overcoming the innate immune response to small interfering RNA,” Hum. Gene Ther., vol. 19, no. 2, pp. 111–124, 2008, doi: 10.1089/hum.2007.179.
[3] S. Filleur et al., “SiRNA-mediated inhibition of vascular endothelial growth factor severely limits tumor resistance to antiangiogenic thrombospondin-1 and slows tumor vascularization and growth,” Cancer Res., vol. 63, no. 14, pp. 3919–3922, 2003.
[4] G. Mahmoodi Chalbatani et al., “Small interfering RNAs (siRNAs) in cancer therapy: a nano-based approach,” Int. J. Nanomedicine, vol. 14, pp. 3111–3128, 2019, doi: 10.2147/IJN.S200253.
[5] R. Acharya, “The recent progresses in shRNA-nanoparticle conjugate as a therapeutic approach,” Mater. Sci. Eng. C, vol. 104, no. June, p. 109928, 2019, doi: 10.1016/j.msec.2019.109928.
[6] M. L. Pecero, J. Salvador-Bofill, and S. Molina-Pinelo, “Long non-coding RNAs as monitoring tools and therapeutic targets in breast cancer,” Cell. Oncol., vol. 42, no. 1, pp. 1–12, 2019, doi: 10.1007/s13402-018-0412-6.
[7] M. Ratti et al., “MicroRNAs (miRNAs) and Long Non-Coding RNAs (lncRNAs) as New Tools for Cancer Therapy: First Steps from Bench to Bedside,” Target. Oncol., vol. 15, no. 3, pp. 261–278, 2020, doi: 10.1007/s11523-020-00717-x.
[8] M. A. Valencia-Sanchez, J. Liu, G. J. Hannon, and R. Parker, “Control of translation and mRNA degradation by miRNAs and siRNAs,” Genes Dev., vol. 20, no. 5, pp. 515–524, 2006, doi: 10.1101/gad.1399806.
[9] A. de Fougerolles, H. P. Vornlocher, J. Maraganore, and J. Lieberman, “Interfering with disease: A progress report on siRNA-based therapeutics,” Nat. Rev. Drug Discov., vol. 6, no. 6, pp. 443–453, 2007, doi: 10.1038/nrd2310.
[10] M. Wang, J. Wang, B. Li, L. Meng, and Z. Tian, “Recent advances in mechanism-based chemotherapy drug-siRNA pairs in co-delivery systems for cancer: A review,” Colloids Surfaces B Biointerfaces, vol. 157, pp. 297–308, 2017, doi: 10.1016/j.colsurfb.2017.06.002.
[11] B. Xiao, L. Ma, and D. Merlin, “Nanoparticle-mediated co-delivery of chemotherapeutic agent and siRNA for combination cancer therapy,” Expert Opinion on Drug Delivery, vol. 14, no. 1. Taylor & Francis, pp. 65–73, 2017, doi: 10.1080/17425247.2016.1205583.
[12] J. K. W. Lam, W. Liang, and H. K. Chan, “Pulmonary delivery of therapeutic siRNA,” Adv. Drug Deliv. Rev., vol. 64, no. 1, pp. 1–15, 2012, doi: 10.1016/j.addr.2011.02.006.
[13] M. Creixell and N. A. Peppas, “Co-delivery of siRNA and therapeutic agents using nanocarriers to overcome cancer resistance,” Nano Today, vol. 7, no. 4, pp. 367–379, 2012, doi: 10.1016/j.nantod.2012.06.013.
[14] M. Saraswathy and S. Gong, “Recent developments in the co-delivery of siRNA and small molecule anticancer drugs for cancer treatment,” Materials Today, vol. 17, no. 6. Elsevier, pp. 298–306, Jul. 2014, doi: 10.1016/j.mattod.2014.05.002.
[15] L. Zhang, Z. Lu, Q. Zhao, J. Huang, H. Shen, and Z. Zhang, “Enhanced chemotherapy efficacy by sequential delivery of siRNA and anticancer drugs using PEI-grafted graphene oxide,” Small, vol. 7, no. 4, pp. 460–464, 2011, doi: 10.1002/smll.201001522.
[16] H. Meng et al., “Codelivery of an optimal drug/siRNA combination using mesoporous silica nanoparticles to overcome drug resistance in breast cancer in vitro and in vivo,” ACS Nano, vol. 7, no. 2, pp. 994–1005, 2013, doi: 10.1021/nn3044066.
[17] P. Eivazy et al., “The impact of the codelivery of drug-siRNA by trimethyl chitosan nanoparticles on the efficacy of chemotherapy for metastatic breast cancer cell line (MDA-MB-231),” Artif. Cells, Nanomedicine Biotechnol., vol. 45, no. 5, pp. 889–896, 2017, doi: 10.1080/21691401.2016.1185727.
[18] J. Majumder and T. Minko, “Multifunctional Lipid-Based Nanoparticles for Codelivery of Anticancer Drugs and siRNA for Treatment of Non-Small Cell Lung Cancer with Different Level of Resistance and EGFR Mutations,” 2021.
[19] Y. H. Yu et al., “Cationic solid lipid nanoparticles for co-delivery of paclitaxel and siRNA,” Eur. J. Pharm. Biopharm., vol. 80, no. 2, pp. 268–273, 2012, doi: 10.1016/j.ejpb.2011.11.002.
[20] J. Gao et al., “Inhibition of hepatocellular carcinoma growth using immunoliposomes for co-delivery of adriamycin and ribonucleotide reductase M2 siRNA,” Biomaterials, vol. 34, no. 38, pp. 10084–10098, 2013, doi: 10.1016/j.biomaterials.2013.08.088.
[21] N. Amreddy et al., “Chemo-biologic combinatorial drug delivery using folate receptor-targeted dendrimer nanoparticles for lung cancer treatment,” Nanomedicine Nanotechnology, Biol. Med., vol. 14, no. 2, pp. 373–384, 2018, doi: 10.1016/j.nano.2017.11.010.