Microparticles (MPs) with smart pH-sensitive macropores have been developed to overcome major technical challenges in oral delivery of biopharmaceuticals. The surface macropores can be employed for direct loading and release of the ingredients in favorable surroundings, which eliminates the concerns about drug denaturation during the carrier fabrication process and guarantees timely release of the drug respectively. The present article summarises our recent progress on MPs response to environmental pH and fabrication process of the MPs to improve their loading capacity and preservation efficiency.
Introduction:
There are several key factors making oral delivery systems the most attractive delivery route for pharmaceutical researchers and industry [1]. In addition to patients’ compliance, oral delivery provides advantages such as a very large surface area for drug absorption (about 250 m2), elimination of hazardous wastes, and applicability for solid drug formulations[1, 2]. Furthermore, the availability of the sticky mucosal layer lining the GI tracts throughout the absorption sites paves the way for drug uptake [1, 3]. However, it should be noted that like any other delivery route, oral systems have also their own challenges. The most important barrier against oral administration may be the harsh acidic environment of the stomach (pH~1.2-2), which can easily decompose/denature the drug molecules [2]. Besides the acidic surroundings, digestive enzymes such as protease and peptidases are abundantly available in stomach [1]. Ingested foods may stay in the stomach for up to 2 hours, which is enough for damaging the native molecular structures of biopharmaceuticals [2]. Consequently, in order for any oral delivery system to successfully deliver the intact drug molecules to the target, it should appropriately protect the drugs inside the stomach and punctually release them inside the target–that is–small intestine. To this end, microencapsulation systems have been developed to address these requirements and to further improve the drug bioavailability.
pH-responsive microparticles (MPs) made of anionic hydrogels with pKa below or equal to the intestinal pH (6.8-7.4) and well above the gastric pH can encapsulate and preserve the pH-vulnerable biopharmaceuticals in unfavorable digestive conditions and release them inside the small intestine [4, 5]. Previously, MPs with smart pH-sensitive surface pores have been developed. The surface pores can be employed for direct solvent-free encapsulation of ingredients in favorable environmental conditions and for timely complete release of them in the target [6]. Since the previous works had clearly shown that a significant loss of drug activity is mainly associated with MPs with incomplete sealed pores during the first few minutes of incubation in gastric conditions, it has been aimed to investigate the initial leakage behaviour of the MPs with pH-responsive macropores in simulated gastric environment in the present study.
1. Materials and Methods
1.1. Fabrication of MPs
MPs in this work were made of anionic copolymers made of methacrylic acid and ethyl acrylate with pKa value ranging from 5.5 to 6, commercially known as Eudragit® L100-55 (hereafter abbreviated as L100). L100 MPs were fabricated through a swelling solvent evaporation protocol previously reported in another study [6]. Concisely, 5 g Eudragit L100 powder was suspended and stirred in 100 ml dichloromethane (DCM) at 300 rpm and 39°C, followed by solvent evaporation steps comprising of incubation at 68°C for 30 minutes and 37°C overnight. The samples were taken after 15min.
1.2. Microstructural analysis of the MPs
The morphology of the MPs was analysed using Scanning Electron Microscopy (SEM), S4800 Hitachi Electron Microscope. The average pore diameter as well as the average particle diameter were measured through 300 direct measurements on the SEM images.
1.3. Encapsulation and leakage tests
FluoSpheres® carboxylate microspheres 100 nm was encapsulated into the L100 MPs through applying vacuum cycles for 5-6 times in vacuum oven. Ingredients-loaded MPs were subsequently freeze dried using AdVantage Pro Freeze-Dryer under the same protocol previously reported [6]. The freeze-drying protocol closes the surface pores, isolating the loaded components from the unfavorable conditions surrounding the MPs. 10 mg freeze-dried MPs from each sample conditions were washed twice: was suspended in 1 ml simulated gastric fluid (SGF), briefly vortexed and subsequently centrifuged at 500g for 2 min. The washed samples were again suspended in 2 ml SGF composed of HCl (0.2 M) and KCl (0.2 M) at pH 2.0. The leakage from each sample condition during the agitation was analysed by fluorescence spectroscopy (excitation: 490 nm, emission range: 500-530 nm) after 0, 5, 10, 15 and 20 min.
2. Results and discussion
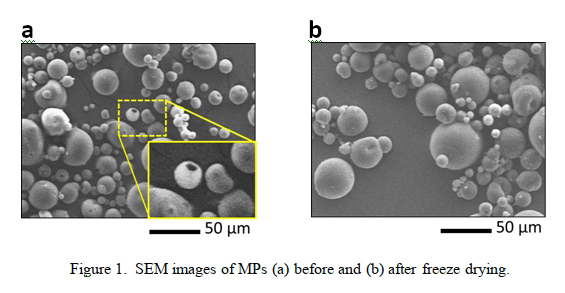
Figure 1 represents the morphology of the MP samples (a) before and (b) after freeze drying. From the comparison of SEM micrographs, it is evident that MPs exhibit smaller average pore diameter after freeze-drying, implying a successful pore closure treatment (compare Figure 1a with 1b). In the swelling process, DCM will diffuse into the polymer powder, which subsequently leads to strong vapor flux during the solvent evaporation step and generates the surface pores [4, 6]. Clearly, larger surface pores pave the way for the loading of larger sized biomolecules. Also, freeze drying can help the pore closure in the MPs through depleting the polymer matrix from water, which in turn intensifies polymer-polymer interactions and subsequently facilitates the migration of the polymer chains to form a homogenous surface [7].
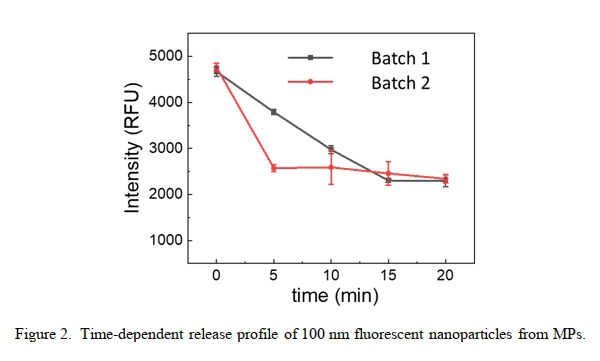
Figure 2 shows the changes in the fluorescence intensity of the fluorescent nanoparticle-encapsulated MPs in the SGF. As shown in the graph, it is interesting to note that the freeze-dried MP sample exhibits steeper decrease in fluorescence intensities over the course of 5-15 min incubation. Since the samples have been washed properly before the analysis, the leakage of 100 nm-sized fluorescent nanoparticles from the freeze-dried MPs can be merely attributed to the loss of ingredients through the incompletely sealed surface pores.In fact, although larger surface pore size makes it possible for the MPs to encapsulate larger sizes of ingredients, it also necessitates more material flow within the polymer matrix to close the surface pores.
pH-sensitive MPs can preserve the components from harsh environments and deliver them to specific targets, making them promising carriers for pharmaceutical and food industries. The most challenging problems associated with these carriers may include: insufficient loading capacity of the MPs, inadequate preservation efficiency of the system, leakage from the MPs under hydrodynamic environmental pressure, delayed and incomplete release of the cargo in the target [7, 8]. In the present study, leakage from the carriers under constant stirring was examined to estimate pore closure efficiency. As far as pored microencapsulation systems are concerned, leakage from the MPs may be ascribed merely to inefficient pore closure of MPs. Ineffective pore closure in turn may originate from two fundamental reasons: 1) large average surface pore size, and 2) insufficient material flow toward the surface pores leading to incomplete surface pore sealing. Clearly, larger surface pores play a critical role in the encapsulation of larger ingredients like large biomolecules and proteins; hence, decreasing the surface pore size may not be a reasonable solution so as to improve the preservation efficiency of the system. The other strategy would be facilitating the material flow within the polymer matrix through decreasing the internal friction to help the chains migration toward the surface pores—that is—increasing the kinetics of the process [9, 10]. According to the thermodynamics, polymer chains tend to move toward the pores to provide a homogeneous surface [7]. As other strategies, annealing the MPs above the glass transition temperature (Tg) of the polymer or swelling the MPs at pH values slightly above the pKa of the polymer may help the pore closure in MPs [4]. It should be noted that annealing the MPs at elevated temperatures (above their Tg) may induce concerns about thermal denaturation of the drugs, especially biomolecules. Swelling the MPs at pH values above their pKa may lead to electrostatic repulsion strong enough to exceed the Van derWaals interactions between the polymer chains and subsequently disintegration or dissolution of the MPs.For successful application of macropored MP-based formulations in oral drug delivery, future research should be focused to develop a method to efficiently control the pore opening/closing behaviour on the MP surface.
3. Conclusion
The stability of drugs in the pored MP system is considerably dependent on the pore closure efficiency. The larger the pores are and the weaker the pore closure efficiency is, the faster the leakage rate will be. However, decreasing the surface pore size may not be a good strategy to address the problems associated with leakage from the MPs, since wider surface pores make it possible to encapsulate larger drug molecules.
References
[1] L. M. Ensign, R. Cone, J. Hanes. Oral drug delivery with polymeric nanoparticles: The gastrointestinal mucus barriers, Advanced Drug Delivery Reviews 64 (2012) 557-70.
[2] H. –J. Choi, J. –M. Song, B. J. Bondy, R. W. Compans, S. –M. Kang, M. Prausnitz, Effect of osmotic pressure on the stability of whole inactivated influenza vaccine for coating on microneedles, PLoS One 10 (2014)
[3] J. G. Werner, B. T. Deveney, S. Nawar, D. A. Weitz, D. A. Dynamic Microcapsules with Rapid and Reversible Permeability Switching. Adv. Funct. Mater. (2018)
[4] S. H. Im, U. Jeong, Y. Xia, Polymer hollow particles with controllable holes in their surfaces, Natur. Mater. 64 (2005) 671-5.
[5] Y. Yun, Y. W. Cho, K. Park, Nanoparticles for oral delivery: Targeted nanoparticles with peptidic ligands for oral protein delivery, Advanced Drug Delivery Reviews 65 (2013) 822-32.
[6] B. Homayun, C. Sun, A. Kumar, C. Montemagno, H. –J. Choi, Facile fabrication of microparticles with pH-responsive macropores for small intestine targeted drug formulation, European Journal of Pharmaceutics and Biopharmaceutics 128 (2018) 316-26.
[7] S. Fredenberg, M. Wahlgren, M. Reslow, A. Axelsson, Pore formation and pore closure in poly(D,L-lactide-co-glycolide) films. J. Control. Release 150 (2011) 142-9.
[8] A. A. Date, J. Hanes, L. M. Ensign, Nanoparticles for oral delivery: Design, evaluation and state-of-the-art, J. Control. Release 240 (2016) 504-26.
[9]A. Kumar, C.D. Montemagno, H-J. Choi, Smart Microparticles with a pH-responsive Macropore for Targeted Oral Drug Delivery, Sci. Rep. 7 (2017) 3059-71.
[10] B. Homayun, A. Kumar, P. T. H. Nascimento, H.-J. Choi, Macropored microparticles with a core–shell architecture for oral delivery of biopharmaceuticals, Arch. Pharm. Res. (2018) 1-13.