Precision medicine is a healthcare customisation platform whereby the personalised perspective involves analysis of patient’s health data to customise the required therapy. This article describes the recent progress in agglomerate design including those prepared from nanoparticles as the therapeutic carrier to meet the required personalised dosage regimen.
Precision Medicine
Drugs can be formulated in the form of tablets, pellets, emulsion, cream and many others for patient consumption. The dosage form development is primarily aimed to promote therapeutic effectiveness and mitigate adverse side effects. Precision medicine encompasses six perspectives: personalised, predictive, preventive, participatory, psychocognitive, and public. The personalised perspective refers to analysis of patient’s health/omics data and customisation of the required therapy. Appropriate drug choice and dosage regimen are designed to derive the best healthcare outcome.
The delivery of a personalised therapy requires the drugs to be dispensed in variable doses in accordance to the health requirement of the patients. Further considerations on polypharmacy and the kinetics of drug delivery to the sites of action of the host are imperative. The dosage form preferably can be mixed and matched to provide the required dose of which is specific to a patient. It is able to carry two or more drugs in a single system, deliver the drugs with the desired kinetics, can possess same or different drug release kinetics, and may engage different drug-specific delivery strategies.Ideally, the dosage form should provide 100 per cent drug bioavailability.
Multi-Particulate Dosage Form
Multi-particulate dosage forms, such as pellets, microparticles and nanoparticles, are vehicles that can be dispensed under the personalised platform through gravimetric measures. A single delivery system can be developed with the required drug and dose. One or more drugs with the same or different drug release and pharmacokinetics profiles can be tailored for the intended application. The nanoparticles have received a widespread interest as drug carrier incancer, diabetes and infectious disease research. The pellets gain the industrial interest owing to their manufacturability, ease of processing and packaging, and availability of large scale manufacturing technology and relevant range of analytical instrument.
The nanoparticles and microparticles can be prepared using spray drying, emulsification, ionic gelation, polyelectrolyte coacervation, milling and homogenisation methods. The pellets can be broadly manufactured by aqueous or melt approach. Melt pelletisation is the recent agglomeration technique. It is an alternative solvent-less method, allowing the formulation of water-sensitive drugs, such as effervescent and hygroscopic drugs, without subjecting them to risks of degradation. The omission of organic solvents, on the other hand, gives rise to a lower cost of production as organic solvents, flame-proof facilities and solvent recovery equipment are not only expensive, but potentially health and environmentally hazardous. The preparation of pellets using melt processes needs no solvent removal. The total processing time is relatively short. Through a judicious choice of molten materials, both immediate- and sustained-release pellets can be prepared.
Granulet
The melt pellets can be produced using high shear mixer. Polyethylene glycol is one critical meltable binder with proven pelletisability. Drug release of a matrix can be promoted by having porous microstructure and/or increasing its polyethylene glycol content. Using high shear technology, the round pellets are nevertheless characterised by low levels of porosity due to high impact, high speed consolidation process. The high impact forces likewise disallow the incorporation of a high polyethylene glycol fraction in pellets to enhance drug release. On this note, Non-Destructive Biomedical and Pharmaceutical Research Centre develops a new processing technique, which produces ‘granulets’: round but porous pellets, for use to develop fast-release solid multi-particulate dosage form (Fig. 1). The centrifugal shear-less circulating air stream is used in replacement of impeller blade to agitate the processing materials. The turbulent air flow intersperses and loosens the assembly of solid particles bound by the molten binding liquid. The agglomerates rollover the wall of processing chamber to spheronise into ‘granulets’.‘Granulets’ satisfies industrial operation such as packaging process due to good flow property. They are porous and can be further added with molten materials to meet the intended drug release kinetics and pharmacokinetics behaviour.
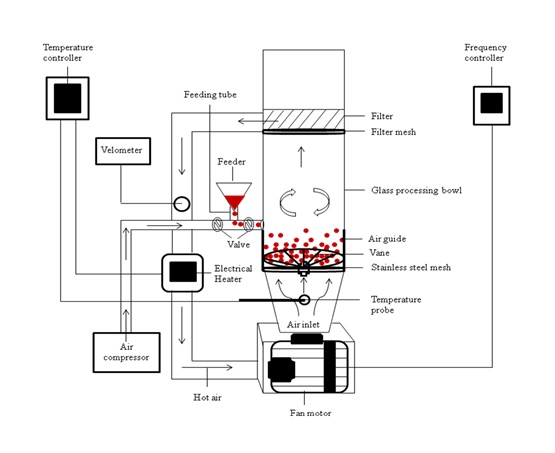
Fig. 1. Centrifugal air-assisted melt agglomeration technology (Wong TW, Musa N. Centrifugal air-assisted melt agglomeration for fast release ‘granulet’ design. International Journal of Pharmaceutics 430, 184-196, 2012).
Sustained/Delayed-Release Pellets
In the case of sustained-release pellets, the high shearing, high impact agitation forces are exploited to consolidate the microstructure of matrix. However, excessively fast agitation speeds result in uncontrollable powder mass movement and heterogeneous pellet growth. Innovative air pressurisation of the processing chamber has been introduced to consolidate the solid particle assembly into pellets, apart from those of agitation forces. Denser pellets are produced with drug release retarded to a greater extent when compared to those produced without air pressurisation.
Alternatively, the sustained-release melt pellets can be prepared through using selective non-meltable excipients. One example is formulatation using alginate and calcium acetate as anionic polyelectrolyte and cationic crosslinking agent respectively, with polyethylene glycolasthemeltable binder. Unlike aqueous pelletisation which produces porous, fast-release pre-crosslinked calcium alginate microstructure, the melt pelletisation process uses no aqueous medium. The reaction between the processing materials during agglomeration is prevented. The calcium alginate crosslinkage reaction only occursin the dissolution phase. During dissolution, a rapid in-situ dissolution of calcium acetate in pellets produces a burst of gas bubbles, fast pellet breakup and dispersion. The dispersed fragments, though are equipped with a larger specific dissolution surface area than the intact matrix, are rapidly crosslinkedby soluble calcium ions from calcium acetate into dense fragments. Their drug dissolution can be retarded and is characterised by an intestinal-specific release pattern where the drug is minimally released till a rise in medium pH.
In Vivo Coated Pellets
Pellets prepared from pectin as core and coat materials are a common approach in design of oral colon-specific drug delivery system. The pectin can be completely fermented by colonic microflora to release the drugs from the pellets. The pectin only experiences partial degradation at pH 2 to 4 of stomach via side chain hydrolysis and at pH 5 to 6 of small intestine via β-elimination of main chain or de-esterification. Nonetheless, it is aqueous soluble. The pellets made of pectin is prone to swelling and erosion in the biological milieu. Premature drug release is expected at the upper gastrointestinal tract, thereby negating the colon-specific drug delivery attributes.
Numerous formulation approaches have been adopted to prevent early drug release in the upper gastrointestinal tract. The pectin matrix has been subjected to crosslinking, coacervation and composite formation and complexation to decrease its drug release tendency. In pellets coated with pectin and hydrophobic polymer, the digestion of pectin results in the formation of pores for drug release. In coat made of complex of pectin and hydrophilic polymers, the pectinolytic enzymes in colon can digest and leach the pectin with drug release thereafter. The leaching of pectin from coat nonetheless may suppress the formation of hydrated pectin channels. The undigested coat can be reconstructed via distension, pore plugging, polymer free volume reduction and drug release is thereby prevented.
In avoidance of inconsistent coat performance in drug release modulation, in vivo coating of pellets is adopted instead of by means of in vitro fluid-bed coating (Fig. 2). In the former, a blend of hydrophilic pectin and hydrophobic ethylcellulose is used as the coating material to pellets made of pectin and loaded with drugs. The pellets and coating material are physically mixed at the solid state in a capsule. Oral administration of the intra-capsular coated pellets is accompanied by coat pectin wetting by the biological fluid and turning adhesive to bind the ethylcellulose onto the pellets. A hydrophobic pellet plug is formed. It is resistant to disintegration and drug release in the early part of gastrointestinal tract. With prolonged transit time, the plug begins to disaggregate. Both coat and core pectins are digested by microbial enzymes in colonic region to release drug.
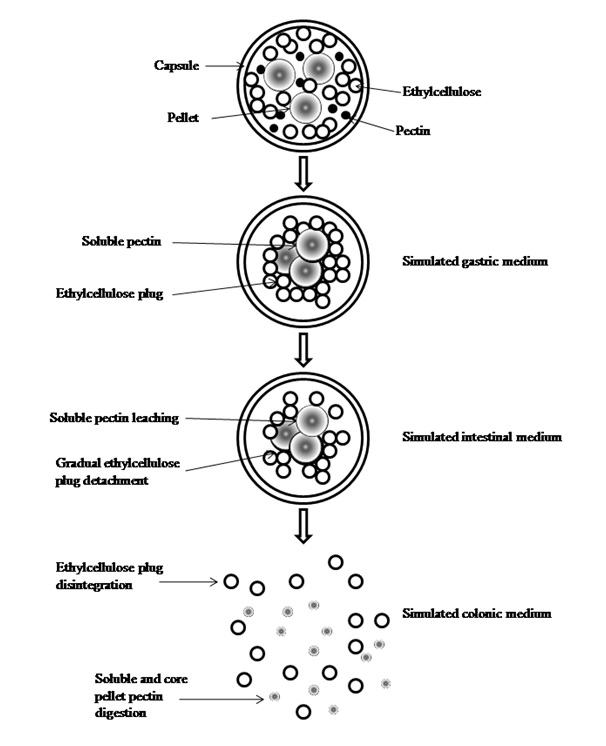
Fig. 2. Fate of in vivo coated pellets with pectin and ethylcellulose in gastrointestinal tract (Elyagoby A, Layas N, Wong TW.Colon-specific delivery of 5-fluorouracil from zinc pectinate pellets through in situ intra-capsular ethylcellulose-pectin plug formation. Journal of Pharmaceutical Sciences 102(2), 604-616, 2013).
Coatless Delayed-Release Pellets
Alginate is a water-soluble polysaccharide made of homopolymeric regions of β-D-mannuronic acid (M) blocks and -L-guluronic acid (G) blocks, interdispersed with alternating structure of -L-guluronic and β-D-mannuronic acid blocks.The pKa value of alginic acid ranges from 3.4 to 4.4, as a function of alginate type and the salt form. Alginate is practically soluble in water.It precipitates as alginic acid in low pH or gastric medium. In a higher pH medium or intestinal fluid, the alginic acid is converted into a soluble gel.The alginic acid ionizes at higher pH and electrostatic repulsion between the polymer chains takes place.The pores of an alginate carrier turn larger to allow small molecule drugs or even large proteins to release.
The preparation of alginate-based colon-specific pellets can be realised via carrier coating. This nonetheless introduces additional steps and costs, and a longer processing time. On this note, Non-Destructive Biomedical and Pharmaceutical Research Centre identifies strategies to design a coatless system through the interplay of processing technology and formulation condition. Melt pelletisation is selected as the processing method of choice. Using melt approach, a high fraction of alginate can be introduced in pellets to retard drug release as the process does not utilise any aqueous medium and pelletisation is not hindered by wetness and cohesiveness of the processing material. The colon-specific coatless alginate pellets are formulated with ethylcellulose, and a combination of moderately water-soluble calcium phosphate and highly water-soluble calcium acetate. Given orally, the calcium acetate dissolves instantaneously to release the soluble calcium to crosslink the alginate chains to prevent burst drug release. The hydrophobic ethylcellulose serves to repel the dissolution fluids from reaching the drug in core throughout the gastrointestinal transit. The crosslinked calcium alginate structure of the pellets is maintained by soluble calcium supplied by the gradually solubilised calcium phosphate throughout transit. The concrete calcium alginate pellets are able to trap the ethylcellulose in the matrix and have it to provide a sustainable hydrophobisation effects.
Nano-in-Micro Soft Agglomerates
Chitosan is a popular matrix of nanocarriers of cancer therapeutics. The chitosan nanocarriers are small in size and hydrophilic. They have a large specific surface area and are prone to premature drug release. Crosslinking and coacervation of chitosan have been adopted to reduce the premature drug release behaviour ofnanocarriers, but to no success due to the specific surface area of the crosslinked and coacervatednanocarriers remains large and responsive to biological fluids.
Nanocarriers have been subjected to microencapsulation to retard drug release. These processes involve impact and/or chemical forces that negate the nanoscale of carriers and their size-dependent biological performances, for example nanocarrier-biological receptor interaction. On this note, soft pellets are designed for the first time by Non-Destructive Biomedical and Pharmaceutical Research Centre where the nanocarriers are agglomerated through depositing on aggregation vehicle of larger sizes followed by assembly of nanoparticles-on-aggregation vehicle into pellets in a dry state under mild agitation forcesand gentle binding action of plastically deformable polymers (Fig. 3). The soft pellets, in comparison to nanocarrier alone, are responsive to the in vivo polymer coating for oral administration purposes. The premature drug release can be mitigated through forming an insoluble plug of soft pellets. The plug survives upper gastrointestinal transit and release the drugs or nanocarriers at lower intestinal tract for local treatment of cancer, inflammation or infectious disease.
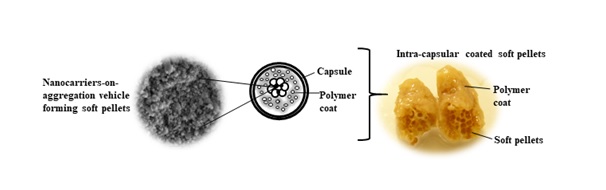
Fig. 3. Soft pellets made of nanocarriers-on-microscale aggregation vehicle and their plug formed in response to in vivo polymer coating (Nafisah Musa, Tin Wui Wong. Design of polysaccharidicnano-in-micro soft agglomerates as primary oral drug delivery vehicle for colon-specific targeting. Carbohydrate Polymers 247, 116673, 2020).
References
1. Nafisah Musa, Tin Wui Wong. Design of polysaccharidicnano-in-micro soft agglomerates as primary oral drug delivery vehicle for colon-specific targeting. Carbohydrate Polymers 247, 116673, 2020.
2. Musalli AH, Talukdar PD, Roy P, Kumar P, Wong TW. Folate-induced nanostructural changes of oligochitosan nanoparticles and their fate of cellular internalization by melanoma. Carbohydrate Polymers 244, 116488, 2020.
3. MdRamli SH, Wong TW, Naharudin I, Bose A. Coatless alginate pellets as sustained-release drug carrier for inflammatory bowel disease treatment. Carbohydrate Polymers 152, 370-381, 2016.
4. Bose A, Harjoh N, Pal TP, Dan S, Wong TW. Drug release, preclinical and clinical pharmacokinetics relationships of alginate pellets prepared by melt technology. Expert Opinion on Drug Delivery 13 (1), 143-154, 2015.
5. Hanafi N, Wong TW. Melt pelletization of alginate: Effects of air pressurization on consolidation and drug release property of pellets. Chemical Engineering Research and Design 104, 479-487, 2015.
6. Elyagoby A, Layas N, Wong TW.Colon-specific delivery of 5-fluorouracil from zinc pectinate pellets through in situ intra-capsular ethylcellulose-pectin plug formation. Journal of Pharmaceutical Sciences 102(2), 604-616, 2013.
7. Wong TW, Musa N. Centrifugal air-assisted melt agglomeration for fast release “granulet” design. International Journal of Pharmaceutics 430, 184-196, 2012.