The development of pharmaceutical drug products involves high variability due to the multifactorial scenario, which at times decreases the batch-to-batch quality consistency. As the 21st century quality initiative by USFDA, the concept of Quality by Design (QbD) has taken enormous importance in attaining the desired quality with enhanced product and process understanding. The present review provides an overview account on the QbD principles and applications in drug product development.
1. Introduction
Pharmaceutical Quality by Design (QbD) is not a buzz word today. From its inception by ICH and USFDA, the concept of QbD has gained significant attention by the global biopharmaceutical industry [1]. The key goal behind QbD implementation was improving the manufacturing standards and to establish a more quality conscious environment for the development of drug products with robust quality and high therapeutic performance [2]. As per the ICH Q8 guidance, the concept of QbD relies on systematic science and risk-based product development using predefined objectives to minimise the challenges like inconsistency in quality, robustness and product performance [3]. For simple to complex drug product development, QbD considers all possible sources of variability during product development exercise, followed by analysing the associated risk and optimisation of the variability to reach target objectives [4, 5].
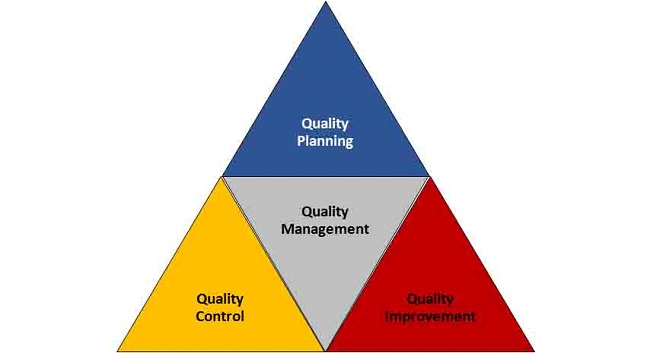
In 1990, J.M. Juran instituted the concept of QbD in the form of ‘Quality Trilogy’, which principally describes the key precepts and perspectives of implementing the quality practices into the product development [6]. Figure 1 portrays the concept of quality trilogy given by Juran. Later, he popularised the ideology of adopting the culture of quality into manufacturing, where ‘quality can be built into the product rather than periodic testing.’ Hence, QbD is also referred as “Quality by planning, but not Quality by Chance”. The applicability of QbD concept has been percolated in diverse manufacturing industries including the pharmaceutical industries [7].
2. QbD: Key elements
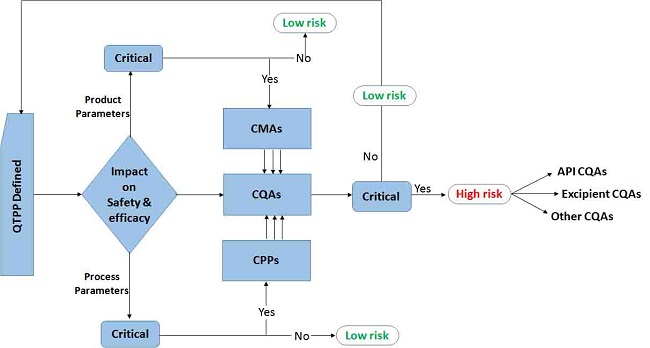
As per the ICH Q8-Q10, implementation of QbD methodology requires thorough understanding of the key elements. Figure 2 pictorially portrays the fundamental elements of QbD as the building blocks for systematic development and optimisation of the drug products and processes.
2.1 Target Product Profile (TPP)
A TPP is a planning tool used to describe the fundamental characteristics related with the safety and efficacy of the drug product [8]. As per the USFDA Guidance for Industry and Review Staff on “Target Product Profile - A Strategic Development Process Tool” published in 2007, it provides detail insight into the TPP elements and its usage for the development and marketing authorisation of the drug products through pre-investigational new drug (pre-IND) application, IND, New Drug Applications (NDA), biologics license applications (BLA) [9]. With this guidance, the agency described advantages of defining TPP for these products, where an applicant or sponsor must “begin with the end goal in mind” or target objectives in mind.
2.2 Quality Target Product Profile (QTPP)
QTPP is considered as a pivotal element of QbD approach, and plays a crucial role in defining objectives of developing a drug product. An ideal QTPP should contain properties of the drug substance, functional traits and clinical perspectives of the target product for the intended patient population [10]. The successful execution of a product development exercise meeting the end objectives always depends on holistic identification of the QTPP [11, 12].
2.3 Critical Quality Attributes (CQAs)
CQAs are considered as the integral components of a drug product, which describes the functional quality traits of a product. It constitutes “physical, chemical, biological, or microbiological property or characteristic that should be within an appropriate limit, range, or distribution to ensure the desired product quality”. The consistency in product performance and process robustness can be easily accessed by monitoring the CQAs [13]. Initially, the Quality Attributes (QAs) are identified from QTPP on the basis of their direct/indirect influence on safety and efficacy of the product, and ultimately on the severity of harm to the patients. Use of prior experience and literature support, at times, help the sponsors and manufacturers for better understanding of the CQAs. Subsequently, out of the possible many QAs, only the vital few are demarcated as the CQAs. With the use of sound science and good quality management system in place, the acceptable ranges of CQAs are defined and monitored throughout the product lifecycle to monitor the product and process performance [14]. From the development perspective, it is always better to investigate the linking of CQAs of the drug product with the formulation and/or process variables. Since multiple functional and nonfunctional elements of a drug product tends to influence the CQAs, hence drug product CQAs can be of various types. These include CQAs associated with drug substance(s), excipient(s), packaging materials, etc. Thus, while identifying the drug product CQAs, one should also analyse possibility of impact of the drug substance or active raw materials and excipient CQAs into consideration.
2.4 Critical Material Attributes (CMAs)
CMAs are the pivotal elements that directly influence the CQAs. These are defined as the physical, chemical, biological or microbiological property or characteristic of an input material that should be within an appropriate limit, range, or distribution to ensure the desired quality of drug products [15, 16]. Initially, the material attributes (MAs) influencing the CQAs are screened first on the basis of their criticality, where only vital few material attributes are identified as the CMAs. These primarily include active and inactive input raw materials or excipients, which possess direct link with drug product CQAs.
2.5 Critical process parameters (CPPs)
Like CMAs, the CPPs are related to the intended process(es) used for manufacturing of drug products and possess direct influence on the CQAs. In another way, these are the key variables which can affect the process performance and variability in product quality [11, 12]. A formal risk assessment approach is used for identifying the criticality of process parameters. With the screening approach in place, only the vital few process parameters are identified as CPPs from the possible few process parameters. Apart from CPPs, the process parameters without having any variability of its impact on CQAs are discriminated as non-critical process parameters (non-CPPs).
2.6 Design of Experiments
Experimental designs are the systematic multivariate tools for optimisation of the drug products and processes using minimum experimentation to unearth maximal outcome [17]. The experimental designs are typically classified as screening designs and optimisation designs. Examples of the screening designs include Fractional Factorial design, Taguchi design and Plackett-Burman design, while examples of the optimisation designs include Box-Behnken design, Central Composite design, Optimal design and Mixture design. Screening designs help in identifying the highly influential factors, whereas the optimisation designs help in optimisation of the factor settings [19]. These experimental designs can be applicable for the development of any type of pharmaceutical products.
2.7 Design Space Considerations
Design space (also referred as proven acceptable range) is a multidimensional combination of relationship between the CMAs/CPPs. When design space is established between CMAs and CQAs, then it is considered as product design space, while design space established between CPPs and CQAs is referred as process design space [20]. The risk assessment, prior experimentations and multivariate factor screening methods are used for identifying criticality of factors and their ranges for establishing a design space. There could be more than one design space in a pharmaceutical product. Ideally, design space is generated using experimental design at lab/pilot scale, and extrapolated to exhibit/commercial scale by establishing correlation with the help of scale-independent parameters [21].
2.8 Control Strategy and Continuous Improvement
A control strategy is designed to ensure consistency in product quality, which is a function of the criticality associated with the input parameters with the output parameters [4, 5, 12]. The elements of the control strategy should describe and justify how in-process control of input materials (drug substance and excipients), intermediates (in-process materials) and container closure system to the final product quality. These controls should be clearly defined at the end of product development and it should be based on the risk-based scientific understanding of formulation and process parameters, thereby leading to continuous improvement.
3. QbD is Omnipresent and Versatile
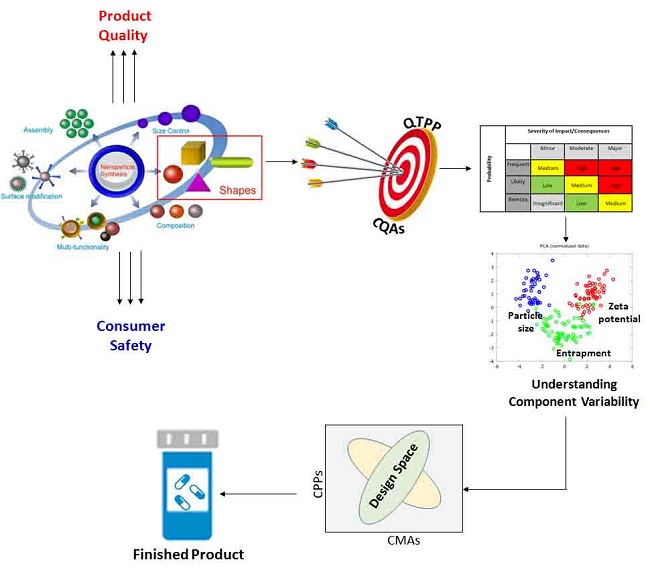
The application of QbD tools in the development of pharmaceutical products is considered as “the best” approach to meet the product quality for the patients benefit. Hence, QbD is omnipresent in the entire product development lifecycle and can be considered as a versatile tool for attaining desired safety and efficacy of the drug products to meet the consumer demand. Figure 3 portrays the flow layout depicting the application of QbD philosophy for holistic development of the drug products. Be it new product or generic product development, QbD is a key quality enabler to accelerate the development with minimal efforts to produce maximal performance. This will certainly provide greater flexibility to the formulation scientist for monitoring product CQAs within the specification during batch manufacturing by avoiding any deviation in the form of Out of Specification (OOS). Besides, as a lifecycle approach, QbD tools allow oversight on the product quality throughout the product lifecycle.
Conflict of interest
Authors declare no conflicts of interest.
References
1. Singh B. Quality by Design (QbD) for holistic pharma excellence and regulatory compliance. Pharma Times 2014; 46(08): 26-33.
2. Yousefi N, Mehralian G, Rasekh HR, Yousefi M. New product development in the pharmaceutical industry: Evidence from a generic market. Iran J Pharm Res 2017; 16(2): 834–846.
3. Martorelli MA. The challenges of drug development. Contract Pharma, 2017. https:// www.contractpharma.com/issues/2017-10-01/view_features/the-challenges-of-drug -development
4. Lionberger RA, Lee SL, Lee LM, Raw A, Yu LX. Quality by Design: Concepts for ANDAs. AAPS J. 2008; 10(2): 268–276.
5. Yu LX, Lionberger R, Johnston G, Olson MC, Buehler G, Winkle H. Quality by Design for generic drugs. Pharm Tech. 2009; 33(10): 1-6.
6. Juran JM. Juran on Quality by Design: The new steps for planning quality into goods and services. Free Press. 1992.
7. Juran JM. The quality trilogy: A universal approach to managing for quality. Quality Progress. 1986.
8. Tyndall A, Du W, Breder CD. The target product profile as a tool for regulatory communication: advantageous but underused. Nat Rev Drug Discov. 2017; 16(3): 156.
9. FDA Guidance. Target product profile — A strategic development process tool. 2007.
10. Singh B, Beg S. Quality by Design in product development lifecycle. Chron Pharmabiz 2013; 22: 72-79.
11. Beg S, Rahman M, Panda SS. Pharmaceutical QbD: Omnipresence in the product development lifecycle. Eur Pharm Rev. 2017; 22(1): 58-64.
12. Beg S, Rahman M, Kohli K. Quality-by-design approach as a systematic tool for the development of nanopharmaceutical products. Drug Discov Today. 2019; 24(3): 717-725.
13. Stangler T. What to control? CQAs and CPPs. European generic medicines association. BWP workshop on setting specifications. London, 2011.
14. Maguire J, Peng D. How to identify critical quality attributes and critical process parameters. FDA/PQRI 2nd Conference. North Bethesda, Maryland, 2015.
15. Chatterjee S. Design space considerations. AAPS annual meeting, Chicago, 2012.
16. Sangshetti JN, Deshpande M, Zaheer Z, Shinde DB, Arote R. Quality by design approach: Regulatory need. Arab J Chem 2017; 10(2): S3412-S3425.
17. Singh B, Raza K, Beg S. Developing “Optimized” drug products employing “Designed” experiments. Chem Ind Digest 2013; 6: 70-76.
18. Beg S, Swain S, Rahman M, Hasnain MS, Imam SS. Chapter 3: Application of Design of Experiments (DoE) in pharmaceutical product and process optimization. In: Pharmaceutical Quality by Design. Beg S and Hasnain MS (Editors), 2019, pp. 43-64.
19. Beg S, Hasnain MS, Rahman M, Swain S. Chapter 1: Introduction to Quality by Design (QbD): Fundamentals, principles, and applications. In: Pharmaceutical Quality by Design. Beg S and Hasnain MS (Editors), 2019, pp. 1-17.
20. Beg S, Akhter S, Rahman M, Rahman Z. Perspectives of Quality by Design approach in nanomedicines development. Curr Nanomed. 2017; 7(3): 191 – 197.
21. Nayak AK, Ahmed SA, Beg S, Tabish M, Hasnain MS. Chapter 18: Application of Quality by Design for the development of biopharmaceuticals. In: Pharmaceutical Quality by Design (Editors), 2019, pp. 399-411.
Figure legend
Figure 1: Quality trilogy for implementing the culture of quality in holistic product development
Figure 2: Key elements and interrelation among them during implementation of QbD
Figure 3: Holistic application of QbD concept for the development of drug products