The number of patient registries is increasing, generating valuable sources of data for holistic disease insights and well-informed therapeutic advances. This article describes findings from a global registry review in sickle-cell disease, assessing their current status as well as future directions that could benefit all stakeholders, including patients.
The value of patient registries in rare diseases
Patient registries are important tools in the healthcare system for systematically collecting data over time from a population living with a certain disease, condition, or exposure [1]. Well-designed and well-executed patient registries hold great potential in providing important disease insights into, for example, patient characteristics, patterns of treatment and care, clinical outcomes and comparative effectiveness, as well as disparities [2, 3].
When it comes to rare diseases, the value of patient registries may be even greater. A number of obstacles can emerge when conducting clinical trials in rare diseases which may impact on the completeness of data collected. For instance, research may be hindered by an incomplete knowledge of the natural history of the condition, geographical dispersion of small groups of patients, or difficulty in capturing clinically significant changes over time [4]. By facilitating the collection of real-world data (RWD) on rare disease populations, patient registries can combat these obstacles. Registries can generate an important resource to improve understanding of the disease pathology and its epidemiology, alongside supporting the development of more targeted regulatory approvals for new therapies and reimbursement strategies [5].
Sickle-cell disease and the global patient registry review
Sickle-cell disease (SCD) is a genetic disease which affects red blood cells, resulting in progressive organ damage and cognitive impairment [6, 7]. Historically, SCD was an often fatal condition that was mainly found in African and Caribbean countries; with time, however, migration has led to cases emerging further afield. Developments in vaccinations and treatments have substantially improved survival rates [6, 8-10].
SCD is classified as a rare disease. Collecting patient-reported outcomes (PRO) – for instance, RWD relating to quality of life (QoL), productivity, and daily disease management – can be a valuable asset to research by providing insight into long-term challenges faced by patients. “Sickle cell disease: a global patient registry review” was published open access in Future Rare Diseases journal, November 2022 [11]. The incentive of the review was to consolidate information relating to SCD registries across the globe, thereby identifying and assessing their key characteristics and highlighting areas for future improvements.
Overview of the SCD registry review
A search for SCD registries, or publications and websites relating to an SCD registry, was conducted using targeted searches of a number of databases and search engines. The searches were not limited by date or location, and a range of registry characteristics were collected, including but not limited to: the current activity status of the registry; the period of data collection; involvement of patient associations; and registry participants’ characteristics (e.g., age, sex, and race/ethnicity). Registry publications and websites were assessed for their availability of information such as participant demographics, treatments, QoL, and the use of PROs.
Thirty-three SCD patient registries were identified, with 64% being active at the time of the review and over three-quarters categorised as national rather than multi-national. National-level registries were most frequent in the US (accounting for over half of registries), Spain, and the UK. Six of the registries were funded by industry. The number of patients included in the registries varied from 62 to over 100,000, the period of data collection varied from 1 to 99 years, and the majority of registries (64%) were initiated in the past 10 years.
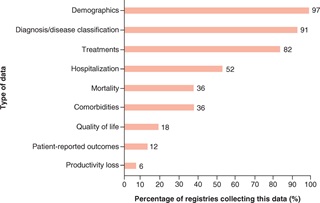
In terms of data capture, the majority of registries provided information on demographics, diagnosis and disease classification, and treatments (over 80% in each instance); data on QoL, however, were captured in only 18% of registries. Only the FISCO Multicenter Registry in SCD Patients, Sickle Pan-African Research Consortium (SPARCO), and Sickle Cell Transplantation Evaluation of Long-term and Late Effects Registry (STELLAR) collected data on PROs, and only FISCO and STELLAR collected data on productivity loss. Three out of 33 registries – the Central Registry of Rare Diseases (CRRD), Get Connected, and the National Haemoglobinopathy Registry (NHR) – reported involving patient advocacy groups (PAGs) in the development process. For two of these registries, PAGs were involved as part of a steering committee.
The past, present, and future of SCD registries
This first review of national and multi-national SCD registries highlighted an increase in initiatives to develop such repositories within the past decade – a finding which is in line with a positive trend in the number of patient registries emerging across therapeutic areas. With a growing recognition of RWD in policy decision making, we anticipate future SCD registries to be funded by industry sponsors who may be seeking evidence to support their reimbursement submissions to a greater extent. It would additionally be of interest to see a wider geographical spread of registries – namely, into Europe, Asia, and Africa – considering the weighting of the national registries identified in this review towards the US.
A noteworthy aspect of this review was its insight into the extent of patient centricity of the included SCD registries. Patient-centric research has moved to the forefront of healthcare in recent years, representing a shift from the role of patients as passive recipients of medical care to their taking an active role in decision-making processes [12]. As such, patient registries offer a unique opportunity for patients’ experience of their condition to be incorporated in research, primarily by providing a source of patient-reported data to complement clinical trial findings. The review evidenced a low number of SCD registries capturing data relating to QoL, PROs, or productivity loss – sources of evidence which are vital to acknowledge the patient perspective and thus develop a holistic picture of real-world disease burden. The value of direct patient reports in validating clinical trial data and capturing meaningful outcomes that may be missed in the highly-regulated environment of the latter has been well-established, and should be considered to a greater extent in future SCD research.
Alongside providing a resource for patient-reported RWD, patient registries can create a space for patient advocacy representatives and organisations to ensure that information that is of the greatest relevance to patients is captured, and in many cases to contribute to and boost recruitment and engagement efforts [12]. As patient registries have traditionally been researcher-generated, the inclusion of patient representatives and organisations continue to be limited in many cases; this is reflected in the findings from this review, which identify only three SCD registries involving patient advocacy groups in their development. Further emphasis on patient involvement in registry design and development in SCD would be valuable, considering the potential benefit to all stakeholders; it is vital, however, to ensure that such collaboration is well-considered so as to effectively connect researchers and patients, and to ensure that the latter’s involvement is not tokenistic [13].
Take home messages
The number of SCD patient registries is increasing, creating the opportunity for the collection of more detailed epidemiological data to demonstrate the burden of SCD, identify shortcomings in disease management, and improve patient care. Future registries should aim to involve patient representatives or organisations in their development, and to include data with further focus on PROs, QoL, and productivity loss, in order to illustrate a more complete view of the disease including the patient perspective.
References
1. Agency, E.M. Patient registries. 2019 [cited 2023 11/01/2023]; Available from: https://www.ema.europa.eu/en/human-regulatory/post-authorisation/patient-registries.
2. Bhatt, D.L., et al., ACC/AHA/STS Statement on the Future of Registries and the Performance Measurement Enterprise: A Report of the American College of Cardiology/American Heart Association Task Force on Performance Measures and The Society of Thoracic Surgeons. J Am Coll Cardiol, 2015. 66(20): p. 2230-2245.
3. AHRQ Methods for Effective Health Care, in Registries for Evaluating Patient Outcomes: A User's Guide, R.E. Gliklich, N.A. Dreyer, and M.B. Leavy, Editors. 2014, Agency for Healthcare Research and Quality (US): Rockville (MD).
4. Professionals, T.A.o.C.R. Common problems in clinical trials for rare diseases. Clinical Researcher 2020 [cited 2023 11 Jan 2023]; Available from: https://acrpnet.org/2020/05/11/common-problems-in-clinical-trials-for-rare-diseases/#:~:text=Obstacles%20Faced%20in%20Conducting%20Clinical,planning%20and%20guiding%20a%20study.
5. Jonker, C.J., et al., Contribution of patient registries to regulatory decision making on rare diseases medicinal products in Europe. Front Pharmacol, 2022. 13: p. 924648.
6. Rees, D.C., T.N. Williams, and M.T. Gladwin, Sickle-cell disease. The Lancet, 2010. 376(9757): p. 2018-2031.
7. Baldwin, Z., et al., Medical and Non-medical Costs of Sickle Cell Disease and Treatments from a US Perspective: A Systematic Review and Landscape Analysis. PharmacoEconomics - Open, 2022. 6(4): p. 469-481.
8. Aygun, B. and I. Odame, A global perspective on sickle cell disease. Pediatr Blood Cancer, 2012. 59(2): p. 386-90.
9. Piel, F.B., et al., Global migration and the changing distribution of sickle haemoglobin: a quantitative study of temporal trends between 1960 and 2000. The Lancet Global Health, 2014. 2(2): p. e80-e89.
10. Chakravorty, S. and T.N. Williams, Sickle cell disease: a neglected chronic disease of increasing global health importance. Archives of Disease in Childhood, 2015. 100(1): p. 48.
11. Borecka, O., et al., Sickle cell disease: a global patient registry review. Future Rare Diseases, 2022. 2(4): p. FRD32.
12. Daugherty SE, L.S., Nowell B et al., The Increasing Focus on the Patient in Patient Registries., in 21st Century Patient Registries: Registries for Evaluating Patient Outcomes: A User’s Guide, D.N. Gliklich RE, Leavy MB, et al. , Editor. 2018, Agency for Healthcare Research and Quality (US): Rockville (MD).
13. Santanello N, L.J., Myers E, et al., Engaging Patients as Partners Throughout the Registry Life Cycle., in 21st Century Patient Registries: Registries for Evaluating Patient Outcomes: A User’s Guide, D.N. Gliklich RE, Leavy MB, et al., Editor. 2018, Agency for Healthcare Research and Quality (US): Rockville (MD).