Adjuvants are immune potentiators administered concurrently with antigens in order to boost vaccine efficacy. The need for vaccine adjuvants was established after discovery of safe, highly purified, but less immunogenic, synthetic antigens via innovation in recombinant DNA technology. Aluminum salts referred to asAlum, have pioneered the field of vaccine adjuvants. However, recent advances in the biology and mechanistic understanding suggest a renaissance era fornovel adjuvants. These new generation vaccine adjuvants broadly work by activating the local innate immune system to trigger antigen specific immune response. In this article, we discuss the history, the current advancements, and the future prospects of vaccine adjuvants.
Introduction
Next to clean water, vaccines have had the most important significance in terms of prevention and treatment of diseases, becoming the most successful medical invention in the past Century. Vaccines have evolved significantly since the development of first successful smallpox vaccine in 1796[1]. Live-attenuated and whole-inactivated vaccines form the basis of most vaccines approved for clinical use. With attenuated or inactivated virus, there is a constant risk of reversion of the killed and/or attenuated pathogen in these vaccines to its virulence form, leading to a pressing need for safer vaccines. Advances in the field of biotechnology and recombinant DNA technology in the early 1980s allowed for engineering of subunit protein antigens[2]. These synthetic antigens offered several prominent advantages over conventional vaccines, such as improved safety, higher yield, improved stability, and lower cost of production[1, 3]. Higher purity and safety of these antigens came with a drawback of being less potent and immunogenic compared to attenuated/inactivated vaccines.
Thus, a need to provide an additional or ‘external’ innate immune activation along with subunit antigens was evident. This led to addition of immunostimulatory components to the vaccine antigen, called adjuvants (in Latin, it means ‘to help’), to enhance immunogenicity of the immunogen [4]. Over the past two decades,research on vaccine adjuvants has grown remarkably, with several new generation of adjuvants being included in licensed vaccine products against infectious diseases such as malaria, influenza, shingles and many more.
Classification of Vaccine Adjuvants
Vaccine adjuvants have evolved in the past Century from only Alum and emulsion based adjuvants administered clinically between 1920s-2000s to next generation adjuvants that include combination of first generation and newer molecular specific adjuvants [5]. Figure 1 illustrates a timeline of key milestones in the development of adjuvants in vaccine products. Vaccine adjuvants are of a diverse family, and hence, cannot be defined using a single integrated structure. They comprise of several naturally occurring or synthetic materials that boost the immunological effect of the antigen. This in return provides advantages such as, antigen dose reduction, cutback on the number of vaccinations, improving the quality of immune response, and in some cases potentially increasing the stability of the final vaccine product [6].
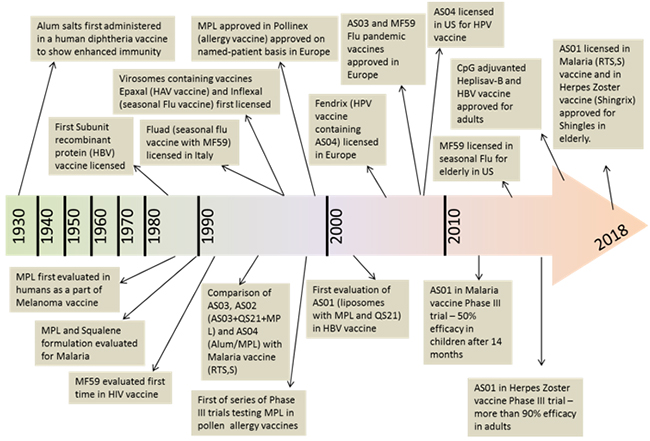
Figure 1.Key developments and milestones in vaccine adjuvantsand a timeline for approval and marketingof the vaccines containing adjuvants. Top of the arrow indicatesthe licensure of vaccines and the bottom of the arrow shows key stages in clinical development.
There are several vaccine adjuvants that are approved and used in commercial productsand other currently in clinical development (Table 1). These vaccine adjuvants can be broadly classified into two main classes based on their mechanism of interaction with innate immune system – First class are adjuvants that effectively present the antigen to the innate immune cells at the site of injection to facilitate rapid uptake of the antigen for enhanced immune response [1]. These first generation are also called as particulate adjuvants; more so because of their physicochemical properties and particle size that would mimic ‘pathogens’ and act as efficient delivery vehicles for antigen also providing for added antigen stability [7]. Alum salts, emulsions, liposomes are examples of this class of adjuvants. Alum salts have been used as adjuvants from as early as the 1930s and are considered as the ‘gold standard’ in the field of adjuvants. Second class would be immune potentiators that provide specific innate immune signals through Pattern Recognition Receptors (PRRs) such as Toll-like Receptors (TLRs). These immune potentiators represent an array of molecules from natural products, to semi-synthetic and synthetic molecules. For most instances, these molecules when injected alone manifest unwanted, and thus undesired, pharmacological effects. The newer generation adjuvants are essentially combination adjuvants that consist of a particulate/delivery vehicle carrying antigen and or immune potentiator.
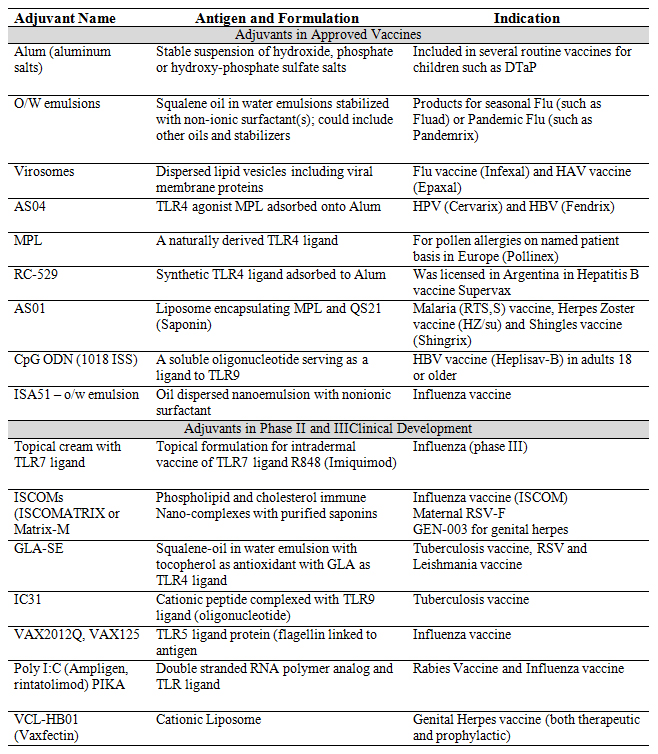
Table 1. Specific examples of adjuvants used in commercial vaccines as well as those in clinical development
C. Mechanismsof Adjuvant Action
Several factors affect the adjuvant immune action once administered, such as antigen-adjuvant interaction, mechanism of adjuvant action, choice of adjuvant, toxicity or local/systemic reaction of the adjuvant. Different adjuvants have several proven or proposed mechanisms of action (Figure 2) to elicit cellular and/or humoral immune response. Thus, prediction of an immune response post adjuvant administration becomes rather difficult. Janeway [8] described adjuvant mechanism of action as ‘immunologists dirty little secret’. Schijns [9] has systematically summarised several theories explaining critical pathways of adjuvanticity that gives an overall understanding of key immunological routes paving a path for novel adjuvant development. These theories explain the general mechanism, however, no adjuvant follows a single pathway of enhancing the immunogenicity. The mechanism of adjuvant action remains a ‘treasure’ that keeps unfolding with advances in research and analytical tools for characterisation. In general, purified vaccine antigens require co-localisation at injection site to facilitate pick up by Antigen Presenting Cells (APCs). Depending on the type of antigen, and adjuvant, spatial and temporal availability of antigen and/or immune potentiator changes and whicheventually iscrucial to achieve desired immune response of sufficient magnitude and quality. Combination of adjuvants (addressed in a later section) that provide synergy in two or more pathways of adjuvant action could provide for a more potent adjuvant. In the next few sections, we have described the current status of vaccine adjuvants prominently used in licensed vaccines.
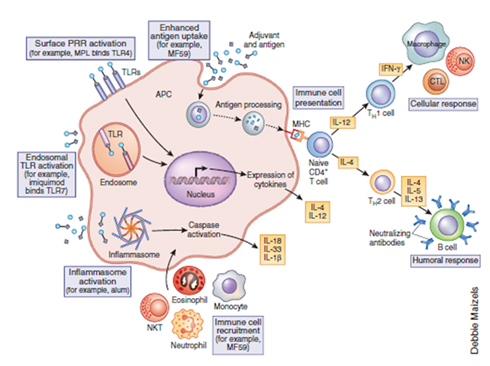
Figure 2.Putative mechanism of action of adjuvants after an intramuscular or subcutaneous injection[10]. Reprinted with permission from Nature Publishing group; Nature Medicine. Reed, S.G., M.T. Orr, and C.B. Fox, Key roles of adjuvants in modern vaccines. Nat Med, 2013. 19(12): p. 1597-608. © 2013
Specific Types of Adjuvants
Aluminum Salts
Around 1920s, in attempt to purify tetanus and diphtheria vaccine antigens, aluminum salts (referred to as ‘Alum’ in this paper) were added to precipitate them from the growth media[11]. Later, it was discovered that these precipitated antigens were more immunogenic than the soluble ones. Since then, Alum-based adjuvants have been used extensively in clinical vaccines providing for a vast database for safety and efficacy of vaccine adjuvants, as well as providing for a gold standard for comparison for all future novel adjuvants[12]. Ironically, very little is known about molecular and cellular mechanism of adjuvant action for Alum, although several theories have only recently started to surface [13]. It was initially hypothesized that Alum enhances the recruitment of immune cells to injection site and hence uptake of antigens by APCs via Nlrp3 inflammasome pathway [14-16] which was debated by several scientists most prominent of which was work from Franchi. et al showing that although Alum produced higher humoral response via nlrp3 inflammasome pathway, its mechanism of action did not depend on it [17]. In general, Alum promotes a strong Th2 adaptive immune response which relates to higher IgG1 antibody, thus producing enhanced humoral immune response. Other mechanisms for adjuvant action of Alum include release of DNA from cell death causing Danger-associated Molecule Pattern (DAMP) recognition, and enhanced phagocytosis by antigen presenting cells [18]. We believe there will be more work on unraveling mechanism of Alum which will significantly help in novel adjuvant research; however, certain pathways like recruitment of immune cells, enhanced antigen presentation, induction of danger signals for innate immune activation and eliciting abundant Th2 response [10]. Alum proved as a successful vaccine adjuvant for several antigens, however, it manifested poor efficacy with certain intracellular pathogens such as influenza virus. Although, Alum alone is still used in some licensed vaccines, in the past 2 decades, advances in adjuvant research has allowed for more potent vaccine adjuvants to be used for more virulent pathogens. Alum has now been used extensively as a delivery adjuvant, to co-deliver antigen and immune potentiating molecules [19] such as monophosphoryl lipid A MPL (AS04 adjuvant used in Cervarix). We will discuss such adjuvants in our later sections. Alum salts, being suspensions in buffers, aren’t inherently the most stable formulations. Additionally, Alum cannot be frozen as it causes significant aggregation of Alum particles, rendering them less effective in adsorbing antigen and/or immunepotentiator [20, 21]. To avoid potential freezing, Alum based vaccines are transported and stored in a very narrow temperature range. Promising proof-of-concept studies have shown the feasibility of lyophilising Alum formulations and could potentially be an alternative to stabilize Alum formulations and create a single vial vaccine making it independent of cold chain distribution [22, 23].
Emulsion Adjuvants
Emulsion adjuvants have also been around for as long as Alum in the form of Freund’s complete (FCA) and Freund’s incomplete (FIA) adjuvants made with mineral oil. The non-degradable mineral oil content of these adjuvants proved to produce detrimental systemic effects when administered in humans [24, 25]. Around 1980s, effort to create oil-in-water emulsion adjuvants with biodegradable oils in attempt to reduce the toxicity and maintain the adjuvanticity began. Squalene oil was abundantly used in several emulsionssuch as MF59 (developed by Novartis Vaccines) being the first squalene oil emulsion adjuvant approved for human use in Europe for seasonal flu vaccine, Fluad [26]. MF59 has been shown to be well-tolerated and safe with millions of doses already administered [26, 27]. Composed of squalene oil and two surfactants - Span85 and Polysorbate80 - MF59 does not interact with antigen or create a depot effect like Alum. Although the exact mechanism of action is still unknown, when injected, MF59 is known to create an immune competent environment [28] at the site of injection which leads to an influx of antigen presenting cells eventually leading to increased uptake of the antigen. The target APC population for MF59 is monocytes and neutrophils both of which aid the translocation of antigen to draining lymph nodes where MF59 additionally primes the adaptive immune response [28-30]. AS03 (a GSK emulsion adjuvant) is another emulsion adjuvant used with pandemic (Pandemrix, GSK) and seasonal flu (Arepanrix) vaccines[31]. AS03 is known to have an additional immunomodulatory component α-tocopherol, which is known to create local and transient inflammatory response enhancing recruitment of monocytes and macrophages to injection site. Morel, et al., have shown that using a hepatitis B antigen, AS03 showed significantly higher titers compared to AS03 without α-tocopherol [32]. However, the exact mechanism of α-tocopherol in providing immune-stimulation is unknown. One of the recent papers [33] sheds light on the downregulation of lipid-metabolism related genes in the draining lymph node which were associated with alteration of Endoplasmic Reticulum (ER) morphology. It was discovered that IRE1-α, an ER stress sensor kinase is a sensor for metabolic changes induced by AS03 in monocytes, that may help in immunomodulatory function of AS03. Finally, both MF59 and AS03 have proven to be immunogenic in children, provide cross protection against mismatched influenza virus strains, and allow for antigen sparing which is important especially in pandemic setting [34]. Emulsion adjuvants have also been targeted to deliver immune potentiators to enhance the potency of the adjuvant and thus, immunogenicity of the antigen [35, 36].
Other Lipid-based Adjuvants
Liposomes are spherical nanoparticles comprising of a phospholipid bilayer with an aqueous core and are used in vaccines for delivery of both antigen and additional immune potentiators. Based on the choice of the phospholipids and other components, liposomes can be used as a carrier by encapsulating in the core, incorporation in the bilayer, or by adsorbing on the surface. CAF01 is one of the oldest cationic liposome-containing adjuvants with Dimethyldioctadecylammonium (DDA) and Trehalose Dibehenate (TDB) is now in development for Tuberculosis (TB) vaccine [37, 38]. Recent formulation advances has allowed for a successful single vial vaccine development using CAF01 and H56, which is a multistage TB antigen by spray drying the two to obtain a single powder that can reconstituted at the time of administration[39]. Another prominent lipid-based adjuvant is ISCOMATRIX [40] comprising of Immunostimulating Complexes(ISCOMS) and QuilA adjuvant (now replaced with refined Saponin preparations). These liposomal adjuvants offer strong CD4+ T-helper cell 1 type immune response as well as high CD8+ immune response [41] More recently, a combination adjuvant from GSK, AS01 a liposome delivering two synergistically acting immune potentiators – QS21 and MPL, was developed for Shingrix, a shingles vaccine in the United States[42].
Immune Potentiators
PRRs like TLRs [43] were found to be activated via Pathogen-asssociated Molecular Pattern (PAMPs) provided by TLR agonists (TLRa). These TLRa molecules are typically present in older vaccines, however resurfaced much later for their immune potentiation activity. This has allowed a better understanding of how adjuvants work, and how preferred immune responses can be induced [44]. One of the first molecules to be identified as a TLRa was Monophosphoryl Lipid A (MPL-A) a lipopolysaccharide from gram negative bacteria. Unfortunately, lipopolysaccharides molecules like MPL from gram negative bacteria [45], are typically large and complex molecules, which bring significant formulation challenges. Recognition of the receptor systems such as TLRs involved in adjuvant immune potentiation pathways has allowed the discovery of Small Molecule Immune Potentiators (SMIPs) [46, 47]. These SMIPs have been used in cancer immunotherapy such as Resiquimod and Imiquimod, applied topically to melanoma cells. Thus, SMIPs can be discovered by applied this knowledge with existing synthetic chemistry to create synthetic small molecules that also offer ease in formulation [48]. Encouragingly, SMIPs have been shown to be more potent than the large biologic molecules [49, 50] and as most immune potentiators, they also have potential for inducing unwanted systemic inflammatory responses when allowed to diffuse away from the site of injection. Hence, a formulation approach is necessary to efficiently deliver the SMIP to local immune cells, while restricting the ability of the SMIP to diffuse from the site of injection [51]. This perceptively led to next generation adjuvants involving delivery vehicles/adjuvants that limit these immune potentiators spatially and temporally to allow for desired adjuvant action.
Combination Adjuvants
These are “next” generation adjuvants that essentially utilize the knowledge from existing adjuvants, mainly the immunological pathways and immune system activation, to create novel combinations that enhance the immunogenicity of the antigen in general [51, 52]. Depending on the adjuvant combination, the overall immune response can be shifted. For example, Alum when used alone promotes a Th2 dominant immune response, however when used to deliver MPL (as in AS04) the there is significant increase in antibody titers as well as the cellular response is skewed towards Th1 [18]. Another one of GSK’s adjuvant AS02 uses the emulsion AS03 to deliver MPL and was shown in a Malaria vaccine antigen study to be more potent than Alum alone, AS03 as well as AS04 [53]. This tells us that based on the inherent disease-targeting antigen and desired immune response, the combination of adjuvants can vary and provide the expected outcome. AS01 is a classic example for not two but three adjuvants – liposomes, MPL and QS21, are delivered along with the antigen. These adjuvants are presumed to act via separate pathways to provide an enhanced immune response. Recently, AS01 was licensed to use in Shingrix in the elderly, a vaccine for Shingles. AS01 has shown significantly higher cellular immune response compared to AS02 when administered with a tuberculosis antigen M72. The frequency of polyfunctional Th1 cells was particularly significantly higher for AS01 groups compared to AS02. In another scenario, Alum poses as the “best delivery candidate” for immune potentiators for several reasons such as vast database of proven safety and efficacy profile, easy to characterize and optimize the formulation [54, 55] and fairly easy to manipulate the molecule and Alum for adsorption purposes. As previously mentioned, Wu,et al.,[56] used the rational design to develop a synthetic small molecule immune potentiator and carefully modified to include the phosphate group for efficient adsorption to Alum facilitating the final Alum/TLR7 adjuvant. Other combination vaccines include E6020 which is TLR4a with MF59. This adjuvant when compared with MF59 alone, administered with meningitis B (MenB) antigens, showed higher bactericidal and serum titers [35]. A recent study with emulsion adjuvants shows the importance of formulation in stabilizing a lipophilic TLR7 agonist and facilitating its formulation with squalene based emulsions to obtain Adjuvant Nanoemulsion (ANE) [36]. These ANE formulations showed higher titers and favorable pharmacokinetic profile of SMIP compared to TLR7 SMIP or ANE alone. Other example of emulsion combination adjuvant is GLA-SE from IDRI, which includes Stable Emulsion (SE) delivering a TLR4a Glucopyranosyllipid Adjuvant (GLA). It has also been successfully shown that GLA-SE can be lyophilized with tuberculosis antigen [57] to obtain a single vial vaccine.
Future Considerations in the Development of Adjuvants
Plethora of published research with adjuvants thus far clearly point at two major considerations: first, the type of immune response elicited by the adjuvants majorly depends upon the co-administered antigen and second, evaluate the need for an adjuvant with the antigen, and then based on the desired immune response, use most appropriate adjuvant to improve vaccine immunogenicity. There are several theories on the mechanisms of currently approved adjuvants. These adjuvants, however, have naturally-derived components that are difficult to characterize and standardize, such as squalene oil (from shark liver) in oil-in-water emulsions such as MF59, AS03, and GLA-SE. Additionally, QS21 which has an active fraction of Quillaja Saponaria tree bark. The focus now should be on finding the synthetic alternatives for these components with inspiration drawn from SMIPs that have shown comparable or even better immune responses compared to naturally derived TLRa. Furthermore, use of high throughput techniques can facilitate the adjuvant research mainly finding novel molecules as well as combinations for targeting TLRs and newer targets such as Stimulator of Interferon Genes (STING), RIG-1-like Receptors (RLRs), and Nod-like Receptors (NLRs). Along with the discovery of novel immune potentiators, as seen with the summary above, formulation of these immune potentiators will play a pivotal role in shaping the immune response. Another key approach in adjuvant discovery should be the idea of simplicity meaning one should ask if the adjuvant is really needed in the vaccine, and if yes, would Alum alone generate the desired immune response. Thus, one should carefully map the desired immune response and use the existing knowledge for further adjuvant/vaccine development.
Conclusions
The development of novel vaccine adjuvants has been rapid in the past two decades and we believe that it will only be uphill from here. There are several unanswered questions [3] which would take several years to be remedied, however, we’re optimistic that the as the underlying immunological principles of adjuvant action unfold, the development of better vaccine adjuvants is evident. Understanding how adjuvanted vaccines affect the immune system in general, can help answer questions about the possible adverse events and the risk/benefit profile for the adjuvant. Advancement in vaccine development programs of mechanism of action studies and systems biology analyses can significantly advocate critical decision making for adjuvant design and selection. Finally, it is imperative to truthfully communicate the vast adjuvant knowledge to mitigate skepticism regarding vaccines and vaccine adjuvants and highlight the possible advantages over safety concerns.
Acknowledgements
The authors deeply appreciate the assistance of Dr. Derek O’Hagan at GSK Vaccines for discussions and assistance with the review of this article.
References
1. Shah, R.R., K.J. Hassett, and L.A. Brito, Overview of Vaccine Adjuvants: Introduction, History, and Current Status, in Vaccine Adjuvants: Methods and Protocols, C.B. Fox, Editor. 2017, Springer New York: New York, NY. p. 1-13.
2. Plotkin, S.A. and S.L. Plotkin, The development of vaccines: how the past led to the future. Nat Rev Microbiol, 2011. 9(12): p. 889-93.
3. O'Hagan, D.T. and C.B. Fox, New generation adjuvants--from empiricism to rational design. Vaccine, 2015. 33 Suppl 2: p. B14-20.
4. Coffman, R.L., A. Sher, and R.A. Seder, Vaccine Adjuvants: Putting Innate Immunity to Work. Immunity, 2010. 33(4): p. 492-503.
5. O’Hagan, D.T., et al., Towards an evidence based approach for the development of adjuvanted vaccines. Current Opinion in Immunology, 2017. 47: p. 93-102.
6. Schijns, V.E.J.C. and E.C. Lavelle, Trends in vaccine adjuvants. Expert Review of Vaccines, 2011. 10(4): p. 539-550.
7. Brito, L.A., P. Malyala, and D.T. O’Hagan, Vaccine adjuvant formulations: A pharmaceutical perspective. Seminars in Immunology, 2013. 25(2): p. 130-145.
8. Janeway, C.A., Approaching the Asymptote? Evolution and Revolution in Immunology. Cold Spring Harbor Symposia on Quantitative Biology, 1989. 54: p. 1-13.
9. Schijns, V.E.J.C., 1 - Unraveling “the immunologist's dirty little secret”, in Immunopotentiators in Modern Vaccines, V.E.J.C. Schijns and D.T. O'Hagan, Editors. 2006, Academic Press: London. p. 1-16.
10. Reed, S.G., M.T. Orr, and C.B. Fox, Key roles of adjuvants in modern vaccines. Nat Med, 2013. 19(12): p. 1597-608.
11. T., G.A., et al., Immunological notes. XVII–XXIV. The Journal of Pathology and Bacteriology, 1926. 29(1): p. 31-40.
12. Malyala, P., et al., The Preparation and Physicochemical Characterization of Aluminum Hydroxide/TLR7a, a Novel Vaccine Adjuvant Comprising a Small Molecule Adsorbed to Aluminum Hydroxide. Journal of Pharmaceutical Sciences, 2018. 107(6): p. 1577-1585.
13. Ennio, D.G., T. Elaine, and R. Rino, Alum adjuvanticity: Unraveling a century old mystery. European Journal of Immunology, 2008. 38(8): p. 2068-2071.
14. Kool, M., et al., Alum adjuvant boosts adaptive immunity by inducing uric acid and activating inflammatory dendritic cells. The Journal of Experimental Medicine, 2008. 205(4): p. 869-882.
15. Eisenbarth, S.C., et al., Crucial role for the Nalp3 inflammasome in the immunostimulatory properties of aluminium adjuvants. Nature, 2008. 453: p. 1122.
16. Martinon, F., et al., Gout-associated uric acid crystals activate the NALP3 inflammasome. Nature, 2006. 440: p. 237.
17. Luigi, F. and N. Gabriel, The Nlrp3 inflammasome is critical for aluminium hydroxide-mediated IL-1β secretion but dispensable for adjuvant activity. European Journal of Immunology, 2008. 38(8): p. 2085-2089.
18. Marichal, T., et al., DNA released from dying host cells mediates aluminum adjuvant activity. Nature Medicine, 2011. 17: p. 996.
19. Garcon, N. and M. Van Mechelen, Recent clinical experience with vaccines using MPL- and QS-21-containing adjuvant systems. Expert Rev Vaccines, 2011. 10(4): p. 471-86.
20. S., S.M., et al., Influence of formulation pH and suspension state on freezing-induced agglomeration of aluminum adjuvants. Journal of Pharmaceutical Sciences, 2012. 101(3): p. 1050-1062.
21. Braun, L.J., et al., Development of a freeze-stable formulation for vaccines containing aluminum salt adjuvants. Vaccine, 2009. 27(1): p. 72-79.
22. Hassett, K.J., et al., Glassy-State Stabilization of a Dominant Negative Inhibitor Anthrax Vaccine Containing Aluminum Hydroxide and Glycopyranoside Lipid A Adjuvants. Journal of Pharmaceutical Sciences, 2015. 104(2): p. 627-639.
23. Clausi, A.L., et al., Influence of protein conformation and adjuvant aggregation on the effectiveness of aluminum hydroxide adjuvant in a model alkaline phosphatase vaccine. Journal of Pharmaceutical Sciences, 2009. 98(1): p. 114-121.
24. Hilleman, M.R., Critical appraisal of emulsified oil adjuvants applied to viral vaccines. Prog Med Virol, 1966. 8: p. 131-82.
25. Murray, R., P. Cohen, and M.C. Hardegree, Mineral oil adjuvants: biological and chemical studies. Annals of allergy, 1972. 30(3): p. 146-151.
26. O'Hagan, D.T., MF59 is a safe and potent vaccine adjuvant that enhances protection against influenza virus infection. Expert Rev Vaccines, 2007. 6(5): p. 699-710.
27. Schultze, V., et al., Safety of MF59™ adjuvant. Vaccine, 2008. 26(26): p. 3209-3222.
28. O’Hagan, D.T., et al., The mechanism of action of MF59 – An innately attractive adjuvant formulation. Vaccine, 2012. 30(29): p. 4341-4348.
29. Seubert, A., et al., Adjuvanticity of the oil-in-water emulsion MF59 is independent of Nlrp3 inflammasome but requires the adaptor protein MyD88. Proc Natl Acad Sci U S A, 2011. 108(27): p. 11169-74.
30. Seubert, A., et al., The Adjuvants Aluminum Hydroxide and MF59 Induce Monocyte and Granulocyte Chemoattractants and Enhance Monocyte Differentiation toward Dendritic Cells. The Journal of Immunology, 2008. 180(8): p. 5402-5412.
31. Garcon, N., D.W. Vaughn, and A.M. Didierlaurent, Development and evaluation of AS03, an Adjuvant System containing alpha-tocopherol and squalene in an oil-in-water emulsion. Expert Rev Vaccines, 2012. 11(3): p. 349-66.
32. Morel, S., et al., Adjuvant System AS03 containing alpha-tocopherol modulates innate immune response and leads to improved adaptive immunity. Vaccine, 2011. 29(13): p. 2461-73.
33. Givord, C., et al., Activation of the endoplasmic reticulum stress sensor IRE1α by the vaccine adjuvant AS03 contributes to its immunostimulatory properties. npj Vaccines, 2018. 3(1): p. 20.
34. Wilkins, A.L., et al., AS03- and MF59-Adjuvanted Influenza Vaccines in Children. Frontiers in Immunology, 2017. 8: p. 1760.
35. Baudner, B.C., et al., MF59 Emulsion Is an Effective Delivery System for a Synthetic TLR4 Agonist (E6020). Pharmaceutical Research, 2009. 26(6): p. 1477-1485.
36. Lodaya, R.N., et al., Stable Nanoemulsions for the Delivery of Small Molecule Immune Potentiators. Journal of Pharmaceutical Sciences.
37. Christensen, D., et al., Cationic liposomes as vaccine adjuvants. Expert Review of Vaccines, 2011. 10(4): p. 513-521.
38. van Dissel, J.T., et al., A novel liposomal adjuvant system, CAF01, promotes long-lived Mycobacterium tuberculosis-specific T-cell responses in human. Vaccine, 2014. 32(52): p. 7098-7107.
39. Thakur, A., et al., Immunological and physical evaluation of the multistage tuberculosis subunit vaccine candidate H56/CAF01 formulated as a spray-dried powder. Vaccine, 2018. 36(23): p. 3331-3339.
40. Drane, D., et al., ISCOMATRIX™ adjuvant for prophylactic and therapeutic vaccines. Expert Review of Vaccines, 2007. 6(5): p. 761-772.
41. Didierlaurent, A.M., et al., Adjuvant system AS01: helping to overcome the challenges of modern vaccines. Expert Rev Vaccines, 2017. 16(1): p. 55-63.
42. Lecrenier, N., et al., Development of adjuvanted recombinant zoster vaccine and its implications for shingles prevention. Expert Review of Vaccines, 2018: p. 1-16.
43. Medzhitov, R., P. Preston-Hurlburt, and C.A. Janeway, A human homologue of the Drosophila Toll protein signals activation of adaptive immunity. Nature, 1997. 388(6640): p. 394-397.
44. Dowling, J.K. and A. Mansell, Toll-like receptors: the swiss army knife of immunity and vaccine development. Clin Transl Immunology, 2016. 5(5): p. e85.
45. Wang, Y., et al., TLR4/MD-2 activation by a synthetic agonist with no similarity to LPS. Proc Natl Acad Sci U S A, 2016. 113(7): p. E884-93.
46. Testerman, T.L., et al., Cytokine induction by the immunomodulators imiquimod and S-27609. J Leukoc Biol, 1995. 58(3): p. 365-72.
47. Wagner, T.L., et al., Modulation of TH1 and TH2 cytokine production with the immune response modifiers, R-848 and imiquimod. Cell Immunol, 1999. 191(1): p. 10-9.
48. Wu, T.Y., et al., Rational design of small molecules as vaccine adjuvants. Sci Transl Med, 2014. 6(263): p. 263ra160.
49. Honegr, J., et al., Structural Properties of Potential Synthetic Vaccine Adjuvants - TLR Agonists. Curr Med Chem, 2015. 22(29): p. 3306-25.
50. Wu, T.Y.-H., Strategies for designing synthetic immune agonists. Immunology, 2016. 148(4): p. 315-25.
51. Brito, L.A. and D.T. O'Hagan, Designing and building the next generation of improved vaccine adjuvants. Journal of Controlled Release, 2014. 190: p. 563-579.