A clinical trial interchange platform can solve complex data integration challenges and also provide enterprise reporting, searching and aggregation capabilities.
The number of solutions available to automate the clinical trials process is increasing at a fast pace with each solution providing a variety of targeted benefits to suit different requirements. Often the same data point is imported into and processed by these different solutions, affecting specific aspects of the clinical trials process in a similar way. Yet, these solutions are most commonly used separately which inevitably lead to time-consuming, costly and unnecessary duplication of documentary data and long-lasting procedures of collaborating information between systems.
Data automation, integration and presentation are required to successfully conduct a clinical trial and it is not uncommon that all these parameters impact one another. Only a platform solution can deliver benefits and features, such as Single Sign-On (SSO), beyond simply transferring data between disparate systems to support a clinical trial.
Traditionally, biopharmaceutical companies have employed various information technology solutions in order to accelerate the clinical trial process, manage data and minimise costs. The most widely used solutions are Clinical Data Management Systems (CDMS), Clinical Trial Management Systems (CTMS), Electronic Data Capture (EDC), Drug Supply Management Systems (DSMS) and Interactive Voice Response Systems (IVRS). Although these systems offer many logistical and commercial benefits, they would be more effective and efficient if they can share information with each other.
The early software solutions tried to tackle this issue through software automation but they didn’t have a standard way to share clinical data. To solve the problem, companies started writing their own custom code to bridge one application with another. To provide an industry standard for describing the clinical data the Clinical Data Interchange Standards Consortium (CDISC) was set up. Unfortunately, the CDISC standard describes a small sub-set of all trial information.
Sponsors and site managers must devote hours every day to keep the different systems synchronised. Typical activities include
For years, drug and medical device developers have dropped information gathered by Case Report Forms (CRFs) into data ‘silos’, i.e., databases designed for only one trial. The main focus being on completing a specific trial as quickly as possible to accelerate the Food and Drug Administration’s (FDA) submission and approval process, the usefulness of raw data in the future has been given less importance. Some vendors of health software attempted to address the integration issues by developing health information solutions which used standardised messages to exchange information within a hospital or region. While basic communication problems have been addressed, these solutions have not achieved complete interoperability and integration of information.
As clinical trial applications became more dynamic and interconnected, vendors started providing standard “hooks” or Application Program Interfaces (APIs) to connect their applications to others. A series of standards evolved to help IT organisations share data across applications and organisations. “Middleware” vendors created software to help define data, connect applications and transfer data more quickly in order to increase productivity, efficiency and customer satisfaction.
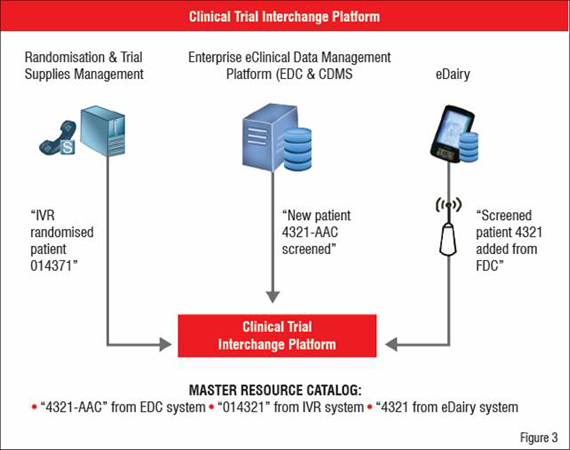
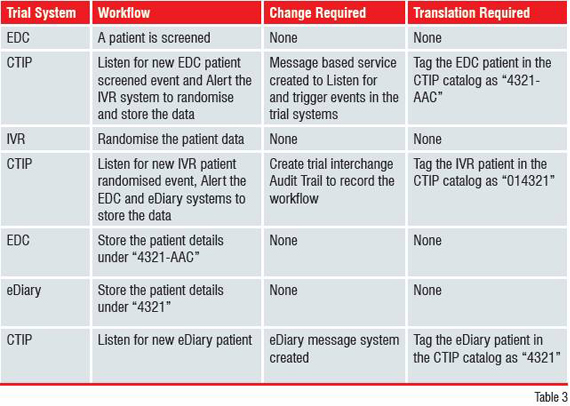
In a typical study, a subject may be screened as patient “4321-AAC” in an Electronic Data Capture (EDC) system, randomised as “014321” in the IVR system and tracked as subject ID “4321” in an eDiary hand-held system. In addition to the same patient being referred to with three different identifiers, the IVR and eDiary solutions share data using different methods; a file-based system and a sophisticated wireless access point respectively.
Collectively termed P2P, this solution aims to share data between two systems by modifying the underlying application code and data on each system for the sole purpose of sending and receiving data, usually through a set of files. Using P2P results in increased trial costs and timelines, more data synchronisation errors, lower flexibility and incomplete decision-support ability. P2P can create more challenges than it solves because:
Using the clinical trial data integration example from above, a P2P approach can be illustrated and summarised as in Figure 1.
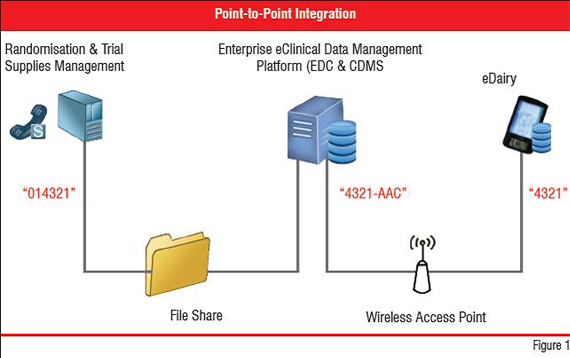
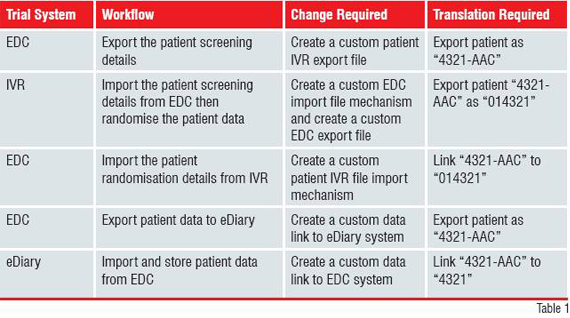
In the case that the three systems have been purchased from different vendors, changes to all of them may not be possible. Further, custom code is needed to manage security between each point. Finally, P2P estabilishes a synchronous workflow that relies on all systems being available to send and receive data through a local file sharing system and the Internet, as well as requiring the EDC system to track the different identifiers representing the patient in each system. This solution is limited, costly to maintain, difficult to support, inflexible and does little to represent the overall clinical trial processes or shared data at decision support level. The High-tech industry has responded by providing solutions designed to solve the shortcomings of manual data entry and P2P approaches. One such solution is Web Services.
The least mature set of technologies available for integrating clinical data, Web Services, enable data sharing between web-based applications irrespective of the underlying platforms or programming languages used.
Web services use Extensible Markup Language (XML), an open standard that was originally designed to facilitate easy exchange of information over the Web. Multiple XML standards have proliferated, but they present serious downfalls impeding easy integration. Many industry-specific definitions are not incorporated into XML. The standard also lacks security, verification and confirmation functionalities necessary for inter-enterprise communication. XML also can’t achieve integration alone.
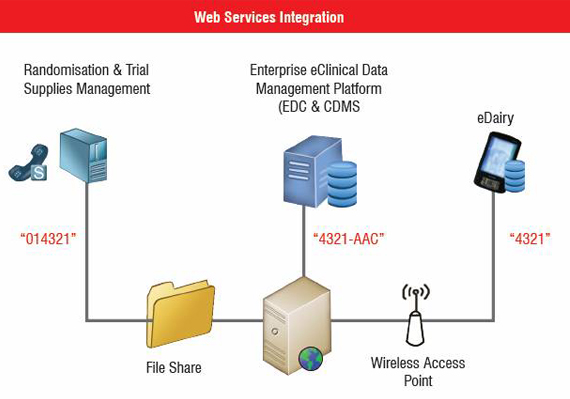
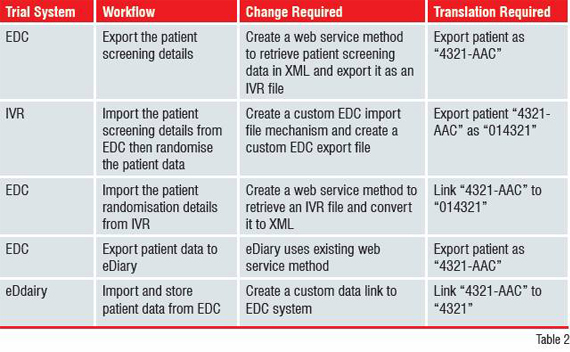
Using the previous clinical trial data integration example, a web service approach can be illustrated and described as as in Figure 2. Just like with P2P, when the three systems have been purchased from different vendors, changes to all of them may not be possible. However, proprietary custom coding is not necessary since web services enforce security and data standards. Web services generate benefits only when all systems are capable of sending and receiving data. Moreover, they require the EDC system to track the different identifiers representing the same patient in each system. A Clinical Trial Interchange Platform (CTIP) solution succeeds where P2P and Web Services fail because it lowers integration costs, time and data errors between internal and external clinical trial systems by serving as a trial interchange to automate processes, share data seamlessly and provide a single decision-support portal for all study resources and activities. It enables all of the solutions used during the trial process to respond to actionable alerts, orchestrate events and report status and results in real-time.
CTIP facilitates unattended asynchronous communication between two or more trial systems. There are instances, however, where synchronous communication is required. As a result, a platform capable of handling both types of communication is needed.
With CTIP, communication between systems is achieved without any system modifications. It therefore allows the different business units to keep operating through the clinical trial life cycle.
CTIP breaks down operational silos with Single Sign-On integration and delivers a holistic solution with a single view of all trial related systems and application dependencies along with server configuration and security information.
CROs or pharmaceutical companies can access, search and organise all study related data and resources in one central location that catalogue the identifiers used in each connected component.
A consistent mechanism helps users to navigate within the central repository and find the required information. Without a platform to route messages and perform automation, compliance enforcement is, at best, labour-intensive, but often, nearly impossible across trial systems. The interchange platform completely automates the task of setting and enforcing regulatory and best practice standards and delivers executive-level, at-a-glance visibility into a system generated audit trail for regulatory compliance. From the previous clinical trial integration example, a CTIP solution can be illustrated and summarised as in Figure 3.
With regards to clinical trial integration, the most important question to answer is whether it is more effective to build integration capabilities into different systems or build a flexible single platform solution. Building a CTIP product would not only make single trial integration easier to implement but also make cross-trial integration a reality. Collecting and combining raw data from different trials can result in billions of cost savings as well as additional revenue. However, formatting data across systems that do not feature integration capabilities can be an extremely labour-intensive task.
Unfortunately, relatively few systems offer the ability to deliver real-time data exchange across multiple platforms and databases, let alone completely different trials. Some solutions can integrate with other proprietary systems, but they do not necessarily work with in-house or other third-party solutions. Implementing a CTIP system designed for multiple trial integration and re-use prevents the need for trial-by-trial development of data schemas, table joins etc. all of which are time-consuming activities and expensive to continually re-invent and validate. However, building a CTIP solution can be difficult.
Other industries already employ flexible, standards-based data models, which the pharmaceutical industry can use as templates to discover whether they are applicable for clinical trial applications. These models can be easily achieved as almost every trial involves the same links between notions such as protocol, patient, treatment group and visit. With traditional P2P single-trial integration systems, every time something changes it impacts on the effectiveness of the integration by potentially upsetting and delaying a lot of the work which has already been completed. When a platform integration approach is implemented, the version of the system being used and its integration capabilities are not interrelated. Data can be imported and exported regardless of their original location. This means that data is collected into a central repository enabling study staff to review all the information at a glance.
Until now, no industry regulations have been enforced to mandate integration of clinical trial systems. Whilst just a recommended approach, CDISC standards have become the preferred choice of many sponsors and vendors. Using a CTIP to gather and manage clinical trial information can help achieve FDA approval. With CTIP, every event occurring throughout the entire trial is automatically captured and stored, eliminating the time-consuming and labour-intensive process of coding each event separately for the FDA audit.
Most biopharmaceutical companies are currently examining the potential benefits of integrating clinical trial data from different electronic solutions and some even making integration capabilities a pre-requisite of vendor selection. Companies need to adopt innovative integration systems, which will provide them not only with the ability to integrate data but also the capacity to automate processes and report status in real-time across different systems and trials. A CTIP approach forms the next stage in clinical trial integration providing important benefits such as cost savings, reduced time-to-market and improved R&D impact. Implementation of such a system can ultimately make products more competitive.
• Kush, Rebecca Daniels, Ph.D. The Cost of Clinical Data Interchange in Clinical Trials, August, 2001. http://cdisc.org/pdf/Cost_of_CDI_in_CT.pdf
• Ferrara, F., Grimson, W., and Sottile, P.A., The holistic architectural approach to integrating the healthcare record in the overall information system. In Proceedings MIE ’99, IOS Press, pp. 847–852. Hughes, Ralph, Multi-Trial Data Integration, Applied Clinical Trials, February, 2005 Harris, Gene G, Cantrell Susan, Enterprise Solutions Integration Technology, March, 2002.