In today’s clinical trial environment, change is at the front lines acting as an obstacle to the success of a clinical supply plan. How many times has the clinical trial design changed after supplies are already packed and labelled? Preparing clinical supplies in this environment is challenging supply chains to create adaptive supplies that can easily respond to the changes in today’s clinical trials.
Whether it is adding new sites in an existing study or adding new doses, clinical customers look to the supply chain to be agile with supplies and respond in turn with flexibility while maintaining timely delivery. There are many challenges facing clinical supplies and how the supply chain can be prepared to meet the demand changes.
Challenges facing clinical supplies
In the past decade clinical trials have evolved to adopt a global approach. Trials today are commonly engaged in multiple countries than ever before and are venturing into new and expanding markets like Asia. This movement has had an impact on clinical supply chain organisations demanding that a common platform of supplies appeal to the needs of a wide scope of countries.
The effects of this demand are several-fold. First is the need to gather translations for clinical labels and incorporate regulatory statements for each country involved in a trial. Secondly, there is the need to source comparators from multiple countries in order to supply the trial worldwide. This process can get complicated by differences in approved sources of material, treatment options, or packaging requirements in each country. Another effect is in the distribution of clinical supplies.
As trials reach multiple countries, import and export regulations, inspections and costs impose the need for companies to dedicate significant resources (internal or external) just to manage these logistics. A factor across all of these elements is the impact of growth and competition in the industry to reach patients in these countries. As new markets gain experience with clinical trials, they adopt new regulations or translation standards, and heighten scrutiny over trial applications and imports. Countries with limited infrastructure can struggle to keep up with all the materials being imported into their country.
As a result of all these effects, there can be a heavy influence on the timelines required to prepare and deliver clinical supplies to sites around the world. A clinical supply chain must be prepared to meet the needs of a trial while facing these challenges of global distribution.
As clinical trials have evolved, there is more emphasis on and deliberation over trial design. It has become a common custom for protocols to be amended several times prior to even the start of the study start and then again during the course of the trial. These changes can range from minor ones, including updates in language or laboratory procedures, to major changes in number of subjects, visit schedules or treatment arms. As a result, clinical supplies are planned based on a theoretical demand of the most likely scenario rather than on a realistic expectation of the needs of a trial. This demands that clinical supplies be agile to adapt to the changes in a trial prior to the start of the study and then again be ready to adapt to changes that occur throughout the trial. One of the most difficult challenges of an ongoing trial is to predict and adapt to enrollment rates. Inclusion and exclusion criteria play a large part in determining how quickly a trial may enroll, but it is also subject to investigator engagement, competition with other ongoing trials, and the overall willingness of the patient population that is required for the study. As a result of poor enrollment, a clinical trial may have to add additional sites or further expand into more countries in order to reach the desired patient population. These changes, if not anticipated, can negatively impact supply stocks.
Recognising that clinical trial design is critical and often varies, growing trends in clinical trials are to utilise flexible dosing paradigms or adopt an adaptive trial design in order to maximise the success of a trial. These designs challenge supply chains to prepare for trials where everything from treatment options to the number of subjects can change at points during a trial. Therefore, clinical supply chains must plan for a supply platform that can adapt to major design changes. To be successful, all variables in a trial must be constantly monitored in order to adapt to trial changes and ensure that demand can be met throughout the trial. To meet ever changing demands, clinical supply chain organisations have to provide:
1. a common supply platform that meets a variety of global needs;
2. a strategic and flexible forecast that is based on a theoretical demand of the most likely scenario; and
3. a supply that continually adjusts to ongoing study variables to ensure that clinical supplies are delivered at the right rime to the right place in the right amount and in the right presentation and still has robust expiry.
Unfortunately there is no secret formula in the industrial vault that always delivers against this expectation. Supply chain project managers can only forecast based on a limited number of variables before the cost of the supply chain overshadows the risk of excluded variables impacting supplies. However, there are many approaches that can be considered to position clinical supplies for success.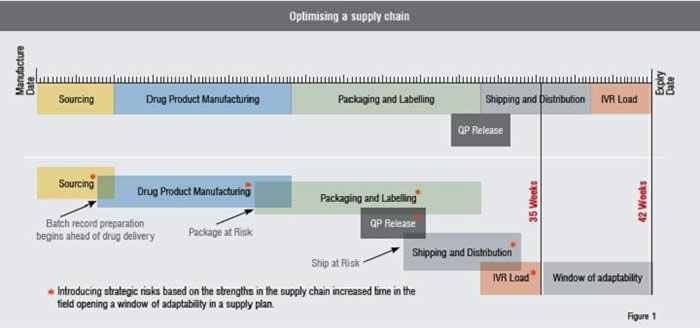
Approaches to meet changing demands
The first approach is to understand and optimise the supply chain. Optimisation requires that a supply chain maximises strengths, provides alternatives for its weaknesses and mitigates its constraints. Optimisation further requires a forecast to minimise assumptions and set clear decision points around key milestones in the trial to evaluate the variables and adjust the forecast accordingly. By increasing the efficiency in which the supply chain can respond to change, a window of time is opened up in which real-time data can be collected from a trial to adjust forecasts and adapt to trial changes. This “Window of Adaptability” is created by reducing cycle times in the supply chain and ensures that clinical supplies always meet the trial demand.
The second approach is to create a common supply platform. This starts in development with formulation. The most valuable tool in a supply platform is to have a single appearance across all dosage forms in a trial. This provides maximum flexibility to meet the demand of a variety of trial designs. This single appearance applies not only to the investigational drug product but also to potential comparator agents. By adopting a common packaging design, supplies can be fit into multiple clinical trial designs, and the supply chain can therefore be positioned to adjust to the changes that may occur. In particular, these strategies are essential for flexible dosing paradigms or adaptive trial design. By having dosage forms with a single appearance in a common packaging design, these materials can be mixed and matched together in a variety of combinations to satisfy a variety of designs without risking the blind of a study.
Part of developing a common packaging platform is developing a global labelling platform. By developing regulatory templates for each country and establishing a library of approved phrases and translations, the supply chain can develop a label that fits with a packaging design for any country ahead of the study demand. By further grouping countries into regional booklet labels, the supply chain is prepared to expand into new countries within any region when enrollment demands it. By combining packaging and labelling platforms together, a single global supply can be generated that is pooled across multiple trials. From a single flexible inventory, multiple protocols can be managed such that when one trial struggles for enrollment, supplies are diverted to another trial that is moving quickly. Managing one inventory helps to reduce the burden on the supply chain to track multiple packaging platforms and study demands so that more attention can be given to monitoring variables and adjusting forecasts for additional supply needs.
Introducing clinical partnership
The most important approach is to create a partnership with your clinical customers. By transforming your customer into a partner, clinical supplies responsibilities are adopted and there is a new sense of co-ownership. Through partnership, key variables which are needed to forecast supplies effectively are identified. Through partnership, the supply chain can contribute to an effective trial design. Working together clinical teams and the supply chain can optimise a packaging platform, fit visit schedules to supplies effectively and identify countries that will perform well for both clinical needs and supply needs..
By selecting preferred distribution points and expanding a trial within a regional label pool rather than across label pools, clinical supplies are positioned for success and the usage is maximised. Through partnership, clinical supplies become a part of the trial design. There is a new sense of visibility of the supply chain which is a key to further developing trust in the partnership. Risks in the supply plan must be disclosed and limitations and constraints highlighted so that the clinical partner understands how changes in clinical design or adoption of certain enrollment strategies will impact clinical supplies.
By offering strengths and Windows of Adaptability to clinical partners, in return the supply chain will be tied into decisions which change the key trial variables. It is important to establish milestones within a Window of Adaptability to discuss enrollment targets and any plans to upgrade enrollment with your clinical partners so that any changes in assumptions fit with the forecasting strategy. When a window is short and the data input is limited, partnership with clinical teams is most important to determine which variables will be included in the forecasting plans and which will be excluded at their own risk. Even when the supply strategy is to provide a bolus supply, partnership is still important to validate that the assumptions and variables used in the initial forecast have not changed and supplies will not expire before the end of the trial. Thus, it is important to communicate with clinical partners during the course of a trial to evaluate assumptions and make decisions that involve clinical supplies.
Adapting to change
Adapting to change is a must in clinical trials. It also places a high demand on supplies to be flexible and be able to respond to multiple variables. By optimising the supply chain, Windows of Adaptability are created which then allow supply forecasts to adjust to real-time trial demand. By adopting common packaging and labelling platforms, maximum flexibility to meet a variety of trial demands could be achieved. The most powerful asset is a good partnership with clinical customers. By changing supplies from an ordering process to become apart of clinical trial design, co-ownership is established and supply plans succeed to meet the demand of a trial.