Clinical trials are pilot studies conducted on human volunteers and patients in a phased manner to evaluate the investigational new drugs developed before introducing into the market. Innumerable registered and well planned clinical trials fail every year regardless of promising animal and preclinical modelling. By the power of the grey star, human sourced stem cells, organoids modelling in the in vitro conditions simulating human systems has the potential to revolutionise the way clinical trials and the phases could be directed in future.
Clinical trials include experiments and the related observations done in clinical settings as part of prospective biomedical research studies on human participants. The studies on human participants are usually designed and performed to answer specific questions about biomedical or behavioural interventions, new treatments, and known interventions/medicines. These studies generate data on safety and efficacy surrounding the medical intervention. The concept of clinical trials has undergone several mutations while modern trials design and principles have precipitated on the importance of randomisation, replication, and factorial experiments.
Clinical trials are conducted on humans while designed to specifically address set questions and broadly to improve health, quality of life. Experiments and evaluations have been part and parcel of medical field, without which there would be no evidence to know the safety and effectiveness of any treatment given to the real patients. In other words, without clinical trials phased and designed as being practised, there is a big risk that patients are given treatments which may not work or which may be harming instead of serving the purpose of treatment.
There are 4 known phases of clinical trials being practised for ages:
Phase 0 is conducted on a small sample size using a small dose of medication under investigation to make sure that it is not harmful to human healthy volunteers before testing higher doses. In this phase, if the test medications effect is different than expected, the study is bound to go back to preclinical research to revisit the functions, re-evaluations.
Phase 1 is conducted on a slightly bigger sample size but again on healthy individuals with key objectives like establishing the highest dose of the medicine tolerated with no serious side effects. Additionally, the best route of administration visa vis the efficacy as well becomes secondary objective of the trial in Phase 1.
Phase 2 is known to involve the patients as subjects but with inclusion and exclusion criteria met as per the design of the trial. Phase 2 is known to involve bigger sample size than Phase 1 while the data collected during this phase supports the strategy and design for Phase 3.
Phase 3 requires patients’ participation in larger cohorts while the purpose of conducting is to evaluate the new medication’s efficacy in comparison to the one already being practised for the same condition. Phase 3 is traditionally double blinded and built on a process called randomisation to help eliminate any bias interpreting results.
Phase 4 is undertaken after the regulatory approval on the use of medication is obtained involving thousands of participants. Phase 4 delivers the new medication’s long term safety and efficacy with study duration lasting for several years.
For long and forever, Phase I studies have been debated and the debate dates back to the history of human experimentation to discover medicines. In Phase 1, the drug is being tested in humans for the first time with no data available on the species and no benefit to the participant. Participants are asked to be willing volunteers to subject themselves as guinea pigs in research, which is questioned and argued by bioethicists on ethical principles as therapeutic misconception. For a healthy volunteer enrolling in a toxicity trial, there is only risk but no medical benefit. Correspondingly, Bioavailability and Bioequivalence (BA/BE) studies are conducted to establish the generic drug’s equivalence to the new drugs and are usually carried out in healthy human volunteers. They are non-therapeutic in nature without any direct benefit to the participants.
“Bioethics and the principles include that we ought not to deceive others, we ought not to harm others or allow harm to come to others, and we ought not to use others as means to an end.” By Christopher K. Daugherty, M.D., of the University of Chicago
Monetary reparation and the supplications to altruism for the benefit of humankind involved as part of the informed consents obtained by the volunteers who are participants are only opportunities for ethical misapplication.
Phase 1 first-in-human studies in Oncology differ from other Phase 1 studies in that they are conducted on cancer patients rather than healthy volunteers. Here, objectives as well alter from the definition of a maximum tolerated dose to the estimation of a recommended Phase 2 dose. Other challenges related to the efficacy and safety profile of novel targeted anti-cancer drugs are conspicuous in Phase 1 for anti-cancer new drugs trial. Likewise, Phase 1 first-in-human studies for progressive Neurodegenerative diseases conducted on human volunteers do not yield data that can justify the design, dose and method of treatment.
Drug repurposing (repositioning or re-profiling or re-tasking) is a new approach for identifying new uses for approved or investigational drug candidates that are outside the scope of the original function attributed.
To determine whether a drug is ready for clinical trials, it involves wide-ranging preclinical studies that produce data on efficacy, toxicity, pharmacokinetics and safety of application. After pre-drug discovery research establishing either target or function discovered, wide doses of the drug candidate are tested using in vitro and in vivo (animal) experiments while insilicoprofiling of the drug–target interactions is an integral tool in the framework. Much like clinical trials, there are certain types of preclinical trials such as exploratory toxicology leading to regulatory studies, and other trials that are specific to the particular question. The only goal of preclinical trials is to move into the clinical stage while the preclinical exploratory and regulatory studies are designed around this goal.
There are broadly five different preclinical model systems in use: insilico, in vitro, ex vivo, in vivo and xenografts based. In all the systems, there are fundamental cell based platforms that have found their spot re-creating the tissue or organ of importance to the extent of humanising animal models. When it comes to cell based platforms, primary cells and transformed cell lines are the only variations put in use. The sources of these cells have been broadly human or animal tissues relevant till date in drug discovery pitch. There are stem cells giving rise to tissue specific cells and tissue derived terminally differentiated cell types known to the researchers.
Stem cells are unspecialised cells of the human body that have the capacity to become any cell of the body with an ability to self-renew. Stem cells are present both in embryos and adult tissues/organs. Totipotent stem cells divide and differentiate into cells of the whole organism. Totipotency is the power to form both embryo and extra-embryonic structures. Zygote is a totipotent cell. Pluripotent stem cells form cells of all germ layers but not extra-embryonic structures, such as the placenta. Embryonic stem cells, Induced pluripotent stem cells are pluripotent cell type examples. Multipotent stem cells can specialise into discrete lineage specific cell types. Haematopoietic and Mesenchymal stem cells are classical examples of this stem cell type. Oligopotent & Unipotent are the types with narrower differentiation capabilities.
Owing to their unmatched properties, stem cells have been found to be acting like base platforms available for multiple genetic diseases, including neurological disorders like Parkinson’s, blood diseases, cardiac syndromes, diabetes and hepatic disorders. The tissue models created can be scaled up to systems that mimic entire organs. Stem cells can be harvested from either patient’s body or healthy donor while transcriptomics and proteomics of the stem cells vary between patient and healthy donor.
Patient sourced stem cells are usually utilised in re-creating disease microenvironment in the petri dish while preclinical stage in vitro assays specific to the disease of interest are performed. Healthy donor harvested stem cells have been trialed and in vitro systems developed are best suited in high throughput screening of chemical libraries, toolbox, exploratory toxicity related testing proto cols, drug repurposing. These in vitro model systems used in toxicity testing are known to have diverse advantages on top of human relevance together with the decrease in the number of animals used for experimenting, the reduced price of maintenance, shortening of the time needed, and increase in throughput for evaluating larger number and their metabolism related data points.
If healthy donor is the source chosen, biopsy as the starting material to harvest stem cells cannot be the ethically accepted mode especially when the preclinical research involves volumes and reproducibility. The only option left to the serious researcher or the industry is to consider human biological discards that have proven credibility as ethically immuned, available in large quantity to access sterile, residing stem cells that are shown to be multi-pluripotent in nature, as the raw material. Human umbilical cord blood, cord tissue, deciduous teeth and adipose tissue are the most popular biological discards qualifying as raw materials to produce stem cell based platforms in petri dish mimicking human physiology. The only way to source this raw material is from the biobanks cryopreserving biosamples as there is no second chance that human life gives to collect these biosamples other than the destined event.
Some of the well accepted in vitro cell based assays in preclinical research that include regulatory need are: Cell viability, Apoptosis, Necrosis, Membrane integrity, Mitochondrial toxicity, DNA damage, Cytokine signatures, Toxicity pathways, Toxicogenomics, Proteomics, Embryotoxicity, Tumorogenicity, Lethal dose, Neurotoxicity, Hepatotoxicity, Cardiotoxicity.
The alternatives to stem cell-based platforms in preclinical research stage that have been (low hanging), tried and tested but not with any specific advantages are: cell lines and animals
Human sourced stem cell based platforms have distinct advantages owing to their origin that is closer to clinical reality, their ability to proliferate in culture conditions simulated in petri dishes, their ability to self-renew to result in volumes that can be managed while retaining Stemness, their ability to differentiate under controlled conditions to other lineage specific cell types like: neurons, adipocytes, osteocytes, chondrocytes, heart cells, liver cells, their ability to humanise 3D models.
Clinical trials in a petri dish or in vitro clinical trials use specimens collected from humans to test how a particular disease will react to a specific therapy or combination of therapies. This strategy can be also used for the development of drugs for specific populations, for precision medicine purposes to predict responses in individual patients or for establishing safety of investigational drug candidates. US FDA has the history of engaging and participating in a public-private partnership involving the Health and Environmental Sciences Institute, the Safety Pharmacology Society, and the Cardiac Safety Research Consortium, multiple global regulators, pharmaceutical companies, and academic laboratories, to develop a comprehensive in vitro proarrhythmia assay with a goal to use a combined in vitro and insilico (computational) testing strategy to predict the risk of drug-induced arrhythmias. This was planned to be part of regulatory clinical trial performed for all new drugs in place of a current clinical trial in drug development - An example for clinical trials in a dish in real time.
Phase 1 of Clinical trials – A compelling case for surrogation in Petri dish
With all the ethical issues surrounding Phase 1 clinical trials where the investigational new drug candidates would be clinically applied for the first time with no data available from clinics, into healthy volunteers, alternative strategies like clinical trial in a petri dish utilising healthy donors-sourced stem cells prepared as platforms for in vitro read outs that can match the clinical settings. The success of the strategy in real time application purely depends on the standardised assays with integrated tools measuring end points evaluating safety related clinical end points.
Transtoxbio - CaseStudy
For pluripotent imagination of user’s mind
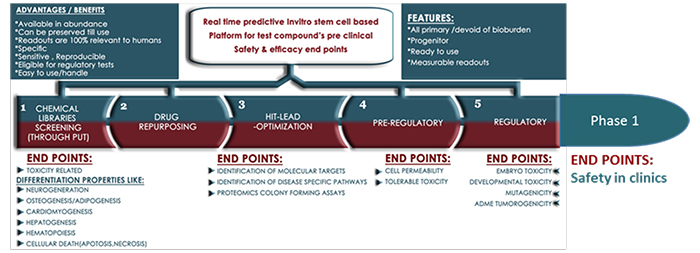
Transtoxbio has all primary, stem cell-based in vitro real time platforms that can predict: biocompatibility, target, functional efficacy, and safety profiles of simple to complex molecules that are being investigated or developed as drug candidates. This real-time platform technology has the potential to support next-generation phenotype based drug discovery (PDD), which is believed to be forward pharmacology. The portfolio’s bandwidth and power to read high throughput screens, phenotype, genotype, proteomics that play crucial role in target identification, pathways recommends integration in preclinical exploratory drug discovery and trials in the labs. Also, some of the platforms of the portfolio can humanise animals in developing human disease models to test the investigational new drug candidates’ efficacy; listing the platform’s wherewithal to participate in preclinical in vivo trials.
Owing to it’s human sourced stem cell compositions, primary with proliferating capacities (to passage and mimic prolonged drug candidate’s exposure time), the platform has the power to read human safety related toxicology specific, measurable endpoints when exposed to lead compounds.
It is the predictive property that answers critical concerns like Embryotoxicity, Genotoxicity, Metabolomics, Transcriptomics, Toxicity related predictive markers (Toxicogenomics), Stem cell cytotoxicity (IC50 on human primary stem cells), Stem cell permeability, Cell distribution of druggable candidates to the extent of mimicking regulatory studies compelling the platform’s adoption in not just preclinics but also in early stages of futuristic clinical trial modalities like that of Phase 1.
The portfolio’s eligibility as surrogate podium to be considered for Phase 1 clinical trial emerges from the following features surrounding the suitability: Source, Abundance of source, Availability and access of the sources chosen, Established protocols in harvesting primary, progenitor cell types either from healthy donor or patient, Producing phenotypically responsive large scale cell based platforms as products that have the power to predict and read the assay end points simultaneously in real time, Bandwidth to access large sample size, Relevance to human species, Not genetically manipulated
Any cell-based platform is of great use for a researcher, user to reproduce the results and obtain meaningful, consistent, statistically significant data output, if it is available in large, batch wise required quantities, which is possible only if the source is available in abundance. Transtoxbio portfolio falls under the category where the source is not donors’ biopsies that has limitations with harvest, yield, and reproducibility; has all new league of human biological discards sourced (ethically immuned) primary progenitor cell-based platforms for speciality next generation invitro to in vivo applications in pre and clinical research.