Pre-clinical trials are the windows of clinical phases in drug discovery and developmental practice. Several invitro, invivo and insilico models have been put to use predicting safety and efficacy of drug candidates to qualify entering into clinical phase. Of these, there are some compelling pre-clinical models like that of human sourced stem cell based platform technologies with advantageous features for better prognosis., if adopted in total would revolutionise the very industry, spend, timelines and success.
Regardless of dedicated research and efforts from academia and industry that have driven the discovery of new treatments for many debilitating diseases, the human race continues to face significant unmet medical needs for numerous health issues. Critical analysis and introspection highlights the differences between human diseases and models of disease in practice, and have noted the failure of the latter to predict. Because of this, there has been a section advocating abandoning models and focusing on clinical trials in human patients; however, ethical hurdles to primary screening of molecules in humans are overwhelming. Medical research continues to remain dependent on model systems for establishing efficacy and safety prior to clinical applications. New approaches to validate cellular and animal models of disease that harmonise their behaviour with human disease are now being aggressively considered. These alternatives include reverse translation of human monogenetic disease to establish homologous cell-based disease models, the use of human sourced stem cells, and molecular fingerprinting of diseased tissues.
The early stage of discovery, sometimes known as pre-discovery, is the founding phase across both drug discovery and development. This stage sets the path to success if assumed honestly by the discovery team. Drug discovery Research & Development(R&D) is known to be a tedious and lengthy process which is predominantly staged and grouped as pre-clinical phase before reaching the consumer market place. Traditional discovery begins with ideation, identification of a target: a mechanism affected by a diseased condition followed by chemistry to meet the targeted needs of the affected mechanism. Proof of concept (PoC) is to demonstrate that the chemistry in discussion is promising and to further verify that the theory has practical potential. Concept and feasibility tests are performed in the early stages of the design and development process. These tasks are elaborate, expensive, and time intensive; however, performing them methodically and on the right, relevant model system(s) is crucial; it can help in mitigating risk to users and preventing the discovery of unexpected failures during verification and validation. By implementing a risk-based approach in the discovery and pre-clinical phase, the value can pay off in revenue and avoiding potential disasters. Unexpected failures can lead to stopping the project, a complete redesign of the product and in worst case scenarios, shelving while reasons could be bad choice of pre-clinical models. Pre-discovery, discovery stages have the need to employ pre-clinical models while the robustness and relevance if is ruthless will lead to pre-clinical experimentation.
There has been an age old belief that simple model systems provide a powerful tool for developing and exploring new therapeutics. But the world of human therapeutics is different from the world of other species as the two worlds make different assumptions while co-existing.
Till date, the hypothesis that if we found drugs that ‘cured’ fly disease models and then successfully showed the same in the mouse model, the drug would magically enter clinical phase with all the approvals and regulations, and was assumed to be the winning recipe. Because it made sense theoretically, 95 per cent of failed trials did not change the deep rooted belief kicking aside the hard truth.
Before testing the drug within a human biological environment, conducting pre-clinical studies on model systems that yield preliminary efficacy, toxicity, pharmacokinetic, and safety information is the routine, reflecting on Why models at all as the question. An increasing change in the drug development pipeline that has emerged in recent years has led to generation of different types of pre-clinical model systems that are surrogates for human disease. Traditionally, the process was limited to the study of drug efficacy in animal models, while the recent discovery and use of CRISPR/Cas9-mediated genome alteration has modernised our ability to generate pre-clinical models containing human pathogenic genetic variants.
Any technology that holds great promise in increasing the correlation between pre-clinical and clinical disease prognosis and treatment translatability calls for compulsive adoption.
There are broadly three pre-clinical model categories: in vitro (test tube or outside normal biological context), in vivo (within a biological entity like an animal), and in silico (computer simulation of the interactions).
In vitro models involve testing a drug outside a living organism, normally on tissues or cells cultured in the laboratory. Initially, these models were built using 2-D systems using tissues or cell lines suspended in petri dishes. However, 3-D models have now been established, with spherical models (constructed using cell lines) and organoids (an artificially grown mass of cells that resembles a particular organ) becoming increasingly popular. The advantages of in vitro models lie in that they can be used easily for large-scale production in pharmaceutical companies. Additionally, the non-requirement of live animals decreases expenses and also dismisses the need for submission of animal protocols in accordance with the AWA (Animal Welfare Act). On a whole, in vitro models present far fewer ethical obligations than animal models do. That said, the absence of a live organism makes it difficult to predict how the drug being tested will interact with multiple organ systems. The lack of physiological exposure means that there is no complex, multicellular response that can be observed. It is also difficult to test the effect of larger, mechanical devices (as opposed to drugs) in an in vitro setting.

In vivo models, on the other hand, involve introducing a drug into a live animal. Animal models used in pre-clinical studies include rats, mice, hamsters, guinea pigs and rabbits and sometimes include calves, swine, and sheep. Pre-clinical studies are devised to test the effect of the drug on the organism as a whole and also on a specific biological function or system. Investigational areas in pre-clinical studies include cardiovascular, endocrine, anti-infective, immune, dermal, musculoskeletal and the central nervous system. These studies are designed to sense a signal that the drug is active on a patho-physiologically relevant mechanism, as well as preliminary evidence of efficacy in a clinically relevant endpoint.
A recent advance in the field of animal models is the use of the xenograft, which involves transplanting a human tissue (typically a tumorous one) into an immune compromised animal such that the response of the human tumour to a drug can be observed without actually testing on humans. Another recent model is using Genetically Engineered Mice where the genes of mice are altered so that the expression of genes involved in tumor transformation or malignancy is modified. These models are declared to be very useful in assessing the effect of gene mutation/deletion on tumour progression.
Using animal models allows for the use of more invasive procedures to assess a drug’s effects, and provides an opportunity for studying the consequences of long-term exposure to a drug. Of course, these advantages come with their own set of limitations, the first being the ethical and legal barriers that are present in animal studies. Additionally, the genetic variability, difference in size, and life expectancy of different animals render them partially unreliable when predicting test results. Finally, it is not always possible to successfully extrapolate the results of an animal study to humans, as the human body may not react to a given substance the same way that animals do.
A model that acts as a compromise between the in vitro and in vivo models is the ex vivo model. It involves taking a tissue or organ from an organism and then studying it in an external environment, with conditions maintained such that it is as similar as possible to the environment within the organism. While an ex vivo model may be more useful than an in vitro model in predicting how a drug might interact in a multicellular environment, it is also disadvantageous in that it is quite difficult and expensive to maintain experimental conditions exactly similar to that of a living organism when studying a tissue outside of the organism. Similar to the in vivo system, it also ethically questionable whether it is right to remove tissues and organs from living animals for experimentation.
One of the other pre-clinical models is the in silico system, which involves screening chemical compounds against biological molecules virtually. This model makes use of large datasets, extensive bioinformatics and computer-based algorithms. Popular modelling methods used include Quantitative structure-activity relationship, and specific statistical approaches that employ experimental data and fragment-based technology. One of its advantages is its time-efficiency, with the ability to screen large datasets of chemical compounds in a matter of hours. Similar to in vitro testing, it also bypasses the ethical hurdles posed by animal testing. Another advantage of computer-based models is that algorithms enable any trends or patterns in chemical or biological behaviour to be identified and documented much faster. This model too has most of the same limitations as the in vitro model. The lack of a physiological environment fails to provide knowledge of the drug’s effects in a larger, multicellular system. Hence, tests in an in silico setting alone are inefficient to determine whether a drug is safe or effective to implement in clinical trials.
While these current models each hold their own merit as discussed, there still remains a large gap between data from pre-clinical trials and actual clinical results. This is particularly true in the field of oncologics, where research has shown that 9 out of 10 attempts to bring a drug from the pre-clinical to the clinical phase fail. This establishes that there is still a dire need for more relevant pre-clinical model systems to be adopted.
“We have moved away from studying human disease in humans. … We all drank the Kool-Aid on that one, me included. … The problem is that [animal testing] hasn’t worked, and it’s time we stopped dancing around the problem. … We need to refocus and adapt new methodologies for use in humans to understand disease biology in humans.” —Dr. Elias Zerhouni., Former US NIH Director.
One of the alternative model systems that Pharma has been resisting is the emerging stem cell-based platform technologies.
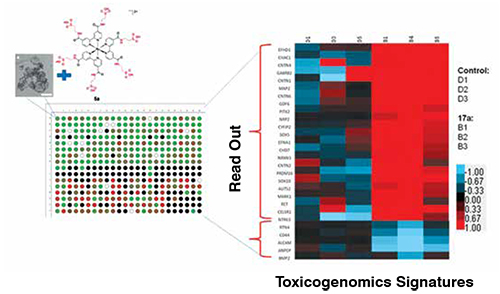
The High Throughput Screening (HTS) activity is part of the drug discovery process, and consists in selecting among thousands of molecules the ones that could have a pharmaceutical use in pre-clinical setting. To do HTS, large compounds libraries or toolbox of molecules are tested on a biological model showing a specific therapeutic target. Human stem cells and their progenies with self-renewable capacity, their ability to differentiate into several tissue progenitors, and their phenotypic responsive nature combined with suitability for Transcriptomics/proteomics define them as a good platform for screening to discover new potential drugs for human diseases. The ability to procure the source, harvest, culture in large scale, batch wise primary progenitors produce make adult stem cells based platforms as the best alternative tools in developing model systems with reproducible, reliable and relevant ones for pre-clinical adoption. These platforms complemented with robots, readers, and a good data mining system, make them a compelling option to replace the irrelevant animal and transformed cell line model systems.
Beyond HTS, stem cell-based approaches, search for novel predictive biomarkers of developmental toxicity, and extend the experimental approach to other tissue specific cellular systems for the prediction of developmental neuro, osteo, hepatotoxicity. Well established differentiation protocols for certain adult cell types most susceptible to chemical-mediated toxicity, any chromosomal abnormality during early development like cardiac, bone, and neural cells have been successfully developed. For neural cell development, stem cells can be efficiently differentiated in vitro into cell types present in the nervous system like mature neurons, astrocytes, andoligodendrocytes, while the process of differentiation acts as a test platform to evaluate neurogenesis, neurotoxicity kind of end points. On the basis of this approach, new rapid and predictive in vitro screens for developmental neurotoxicity testing have been developed.
Even in stem cell based model systems, there are two fundamental criteria like potency and unlimited source availability that make them suitable for investigations spanning traditional discovery to regulatory testings. New exciting avenues of research on the role of microRNA (miRNA) in toxicogenomics and the possibility of epigenetic effects on gene expression opens the possibility to discover new molecular endpoints that might contribute to a further understanding of chemical-mediated developmental toxicity on stem cells based model systems.
Furthermore, in line with new directions for toxicity testing, in the light of advances in understanding biological responses to chemical stressors involving the mapping of toxicity pathways in differentiating human stem cells and identification of critical pathway perturbations that represent molecular initiation events for adverse effects, stem cell-based model systems are the only choice in both exploratory and regulatory testings in pre-clinical phase.
www.transtoxbio.com portfolio of stem cell based products, MatTek’s tissue models, XCellR8, Reprocell’s Biopta are some of the global companies offering alternative pre-clinical model systems and based services as offerings to pharmaceutical research, professional toxicity testing labs. Each of the company’s products/services are distinct in this space serving the need of the hour to support predictive prognosis of the drug pipeline.