Human challenge trials are a useful approach in the establishment of early efficacy for vaccines / therapeutics for infectious diseases. This article will describe how they can be used as a strategic tool for establishing an early risk-benefit and guide and enhance the success rate of future clinical development.
Although slowly fading away from the collective memory, the SARS-CoV-2 pandemic that started in early 2020 and the unprecedented health crisis on a global scale that followed, reminded us about the necessity of pandemic preparedness.
Pandemics are not new to humanity. Throughout history, mankind has been confronted with pandemics on a regular basis. Two well-known examples are the bubonic plague, better known as the Black Death, that ravaged large parts of Europe in the 1300s killing between 25 and 75 million people, and the “Spanish flu” influenza pandemic killing 40–70 million people worldwide shortly after the First World War.
In the second half of the 20th century, a number of less severe pandemic influenzas emerged in 1957–58, 1968, and again in 2009. In each instance, influenza vaccines targeting specifically the circulating virus were developed, although the scientific community is still debating how effectively the vaccines curtailed the disease spread. Also tropical diseases can cause a pandemic, as illustrated by the 2014–2016 Ebola outbreak in west Africa caused over 28,000 cases and 11,000 deaths.
Vaccines and antivirals are a critical part of our arsenal against infectious diseases,Their development however is a challenging, lengthy and expensive process with a high attrition (Gouglas et al., 2018).
Establishing an early risk-benefit profile for a vaccine or antiviral under development is essential to guide the future strategy and speed up time to market. Human challenge studies (HCT) or Controlled Human Infection Models (CHIM) are useful tools to assess early efficacy.
In human challenge studies, healthy volunteers are administered a well-characterised pathogenic or virulent strain of a challenge agent. This can be a virus (i.e. influenza), bacteria (i.e. cholera) or a parasite (i.e. malaria) depending on the trial.
Challenge studies are not a novel concept. Already in the 19th century, Louis Pasteur conducted challenge trials where chickens were challenged with a weakened bacteria causing chicken cholera, immunising them from further chicken cholera infection. In the United Kingdom, HCTs have been performed since 1946 when the Medical Research Council established the Common Cold Unit (CCU) — also known as the Common Cold Research Unit (CCRU) — at Salisbury, Wiltshire. This unit was set-up to research common colds in view of reducing their human and economic costs (Metzger et al., 2019; Roestenberg et al., 2018).
Since the 1990s HTCs have found their way into clinical development, and are used to provide early performance data through proof-of-concept (PoC) trials and to establish the mode of action (MoA) of vaccines and antivirals. Governmental and commercial organisations are developing various challenge models to accelerate vaccine development programmes in respiratory tract infections like influenza and RSV. They are also used to gain a better understanding of the underlying pathological processes that drive immune responses. HCTs were first mentioned in regulatory guidance in 2010.
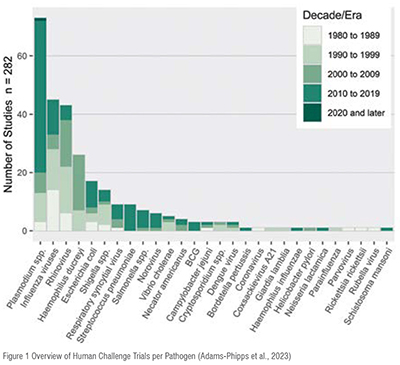
Challenge trials have been performed in a great variety of infectious diseases as illustrated in the table below, including, recently, a COVID-19 challenge model (Killingly et al. 2022). (Figure 1)
In a CHIM trial a well-characterised strain of an infectious agent (virus / bacteria / parasite) is given to carefully selected adult volunteers after they have been vaccinated or before they receive the antiviral to be tested. They follow a traditional double-blind design comparing the treatment group with a placebo control group. These trials are performed in specialised quarantine units, where the inoculated volunteers are under 24/7 medical supervision. A schematic overview of HCTs involving a vaccine and an antiviral can be found below: (Figure 2) (Figure 3)

In current drug / vaccine development, human challenge trials can be used in a variety of ways, but the most common is for proof-of-concept (PoC) studies. Due to the reduced number of subjects needed for a challenge trial (40-60 subjects) compared to a classic `field` PoC trial (180-600 subjects), HCTs provide a cheaper alternative. In addition, their shorter duration allows for a quicker availability of efficacy data.
The primary objective of POC trials is to provide early evidence about efficacy and to establish the likelihood of a drug being successful in later clinical trial phases. Hence, PoC studies allow drug developers to make smarter and data-driven "go or no-go" decisions about continuing with the clinical development and start with larger, more expensive clinical trials.
They can also be used as dose-finding studies, testing different dosing regimens or vaccine / adjuvant combinations to decide which combination to move forward to field trials. This approach is being used extensively in the down-selection of malarial vaccine candidates and is now an integral part of the malaria vaccine development cycle (Sauerwein et al..2011) — In the development of Mosquirix (RTS,S) for example, a phase 2 malaria human challenge trial was used not only to show early efficacy, but also to test different vaccine adjuvant regimens. (rationale: a new malaria vaccine has been approved recently) This enabled the initiation of phase 3 field trials conducted in over 15,000 infants and young children in Africa (RTS SCTP, 2015) and the ultimate market approval of the vaccine.
The results of point of care (PoC) studies are pivotal for strategic decisions in early drug clinical trial development and help the design of later stage field trials (Baay et al., 2019; Wildfire, 2021). Being able to establish an early risk-benefit profile based on phase 1 safety data (`risk`) and HCT efficacy data (`benefit`) can guide further finetuning by taking a data driven approach, and deciding which candidates or treatment regimens to bring to field trials. This reduces the risk of `late stage` failures and focuses time and resources on vaccines and treatments that are more likely to show success in phase 3 trials.
Data from CHIM trials have been accepted by regulators as supportive for the following:
The World Health Organization (WHO) has published guidance on the use of challenge trials in vaccine development (WHO, 2016; WHO, 2017; WHO, 2020a; WHO, 2020b). The guidance also specifies that if they are performed, human challenge trials may be of particular use:
HCTs can play a unique role in resolving a number of the issues faced in the development of a future `pandemic vaccine`, like the recent SARS-CoV-2 pandemic.
The first advantage is that fewer subjects are needed for a CHIM (80 – 120) than for a classic clinical field trial (200-400). Also the inclusion criteria for the study group can be limited to subjects at the lowest possible risk, ie, healthy 18 to 25-year-olds with no comorbidities. Secondly, challenge trials can be used to establish the infectious dose of the pandemic virus, starting from a very low dose and uptitrating in a classic Single Ascending Dose design. Additionally, CHIMs can be used for side-by-side comparison of vaccines, limiting the need for placebo controls, and therefore reducing potential ethical burden. They can also be used to determine correlates of protection, which could in turn be used to better design Phase III studies. Finally, if the CHIM results are favourable, such a trial can be used to support an emergency use authorisation application, for example for use in high-risk populations (Baay et al., 2021; Rapeport et al., 2021).
Early interaction with regulatory agencies including the European Medicines Agency (EMA), Food and Drug Administration (FDA), etc, via a scientific advice meeting or a pre-IND meeting is strongly recommended to discuss the implementation of a Human Challenge Trial in any development programme.
Another important issue are the regulatory and operational aspects of running a human challenge study itself. It is extremely important to avoid cross-contamination between the patients infected with the virus on one side and the study staff on the other side. The aim is to avoid the virus from being spread to the “outside world” and to avoid infected study staff infecting the patients and potentially jeopardising the study results by infecting placebo patients. Before enrolling into a HCT, volunteers undergo a battery of screening test, including a serosuitability test to ensure potential volunteers don’t already have antibodies against the challenge agent being used.
Participants are isolated in a specifically designed quarantine unit and will be treated according to the principle of “reversed-barrier nursing.” This method is very similar to barrier nursing used in an intensive care unit (ICU) setting, where the aim is to keep pathogens away from the ICU patients by creating a barrier between the outside world and inside a patient room by using gloves, masks, gowns, and disinfectants. The same principle also confines the challenge agent to the facility and prevents it from spreading into `the outside world`. Depending on the pathogen used subjects can remain in quarantine for up to two weeks.
HCTs play a very important and supportive role in both our understanding of disease and the testing of novel antivirals and vaccines. Animal models for many diseases are poor at predicting disease pathogenesis especially for human host restricted diseases. Properly designed and ethically conducted CHIM studies have tremendous potential to improve our understanding of pathogenesis, help design better vaccine candidates, reduce the costs and timelines of vaccine / drug development by providing early insights in the efficacy and dosing / administration regimens.
In combination with phase 1 safety data, this establishes an early risk-benefit profile to further guide the development of the vaccine / antiviral.
As illustrated by the Vaxchora approval, human challenge trials can be used as the `pivotal efficacy` efficacy element in a marketing authorization application. A similar approach can be envisaged in a regulatory pathway for accelerated approval for any future pandemic vaccine.
References
Adams-Phipps et al. A Systematic Review of Human Challenge Trials, Designs, and Safety. Clinical Infectious Diseases (2023)
Baay et al. Human Challenge trials in vaccine development. Biologics (2019)
Baay et al. Controlled human infection to speed up SARS-CoV-2 vaccine development Frontiers in Immunology (2021)
EMA. Vaxchora EPAR EMA/82271/2020 (Available at Vaxchora | European Medicines Agency (europa.eu)) (2020)
FDA.: Vaxchora information. (Available at: VAXCHORA | FDA)(2016)
Gouglas D et al. Estimating the cost of vaccine development against epidemic infectious diseases: a cost minimisation study. Lancet Glob Health (2018)
Killingly et al. Safety, tolerability and viral kinetics during SARS-CoV-2 human challenge in young adults. Nature Medicine (2022)
Metzger et al., Experimental infection in humans – historical and ethical reflections. Tropical
Medicine & international Health (2019)
Rapeport et al., SARS-CoV-2 human challenge studies — establishing the model during an evolving pandemic. New England Journal of Medicine (2021)
RTS SCTP Efficacy and safety of RTS,S/AS01 malaria vaccine with or without a booster dose in infants and children in Africa: final results of a phase 3, individually randomised, controlled trial. Lancet. 2015
Roestenberg et al. Experimental infection of human volunteers. Lancet Infectious Diseases (2018)
Sauerwein et al. Experimental human challenge infections can accelerate clinical malaria vaccine development. Nat. Rev. Immunol. 2011
WHO. Human challenge trials for vaccine development: regulatory considerations. 2016.
WHO. Guidelines on clinical evaluation of vaccines: regulatory expectations. (2017)
WHO. Key criteria for the ethical acceptability of COVID-19 human challenge studies.
WHO Working Group for Guidance on Human Challenge Studies in COVID-19, 6 May (2020a).
WHO. Feasibility, potential value and limitations of establishing a closely monitored challenge model of experimental COVID-19 infection and illness in healthy young adult volunteers. Report from the
WHO Advisory Group on Human Challenge Studies, 2 December (2020b)
Wildfire. Modelling the world - can deliberately infecting healthy volunteers really tell us much about what happens outside the clinic during an epidemic or pandemic? Drug Discovery Today (2021)