Digital technology is increasingly used to improve clinical trial efficiency. Its use spans all phases of drug development, and impacts areas such as recruitment and retention of patients, wearables, virtual clinical trials, and health economics outcomes research. Leveraging emerging data sources and technology can reduce development costs and time to market.
Clinical trial development has evolved significantly over the past few decades. Digital technology is increasingly used to improve clinical trial efficiency. Its use spans all phases of drug development, and impacts areas such as recruitment and retention of patients, wearables, virtual clinical trials, and health economics outcomes research. Leveraging emerging data sources and technology can reduce development costs and time to market.
Reports of clinical trials pre-date the digital era, with James Lind being credited by many to have conducted the first controlled clinical trial in 1747 (Collier 2009).This study involved rudimentary data collection from 12 sailors with scurvy, who were assigned to one of six different forms of treatment. The men who were treated with citrus recovered after 6 days of therapy, whereas, the other treatments had no demonstrable effect.
Although the number and nature of successful clinical trials proliferated in the ensuing years, the adoption of digital record keeping during the industrial revolution introduced key efficiencies into the data collection and analysis required for drug development.
A critical element of trial design is ensuring the study population is accurately diagnosed with the indication of interest, and that any biomarkers predictive of response to treatment are correctly identified. Electronic health records are real-time, digital, patient-centred records that can be created and managed by several providers. Anonymised electronic health record databases permit strategic searches to evaluate and quantify the impact of protocol specific inclusion/exclusion criteria on the patient recruitment potential of a given clinical trial protocol. They are used during protocol design and can also be informative in the event of protocol amendments that involve eligibility criteria.
A specific example relates to the use of various computer-based cognitive assessments to monitor cognitive health. This data, when confirmed to be psychometrically valid, is critical in selecting patients appropriate for new therapies in Alzheimer’s disease and other neurodegenerative disorders. Computerised tests can be administered remotely, and in many cases, are more sensitive than paper tests.
Trial sponsors and contract research organisations have also gradually increased the adoption of electronic informed consent and EDC systems to collect electronic patient-reported outcomes (PROs). A PRO is any report of the status of a patient's health condition that comes directly from the patient, without interpretation of the response by anyone else. Per the U.S. Food and Drug Administration’s (FDA) guidance, clinical trials using reliable PRO instruments may be used to support medical product labelling claims. In addition to paper-based PRO instruments being replaced by electronic versions, there has been increased use of data from wearable devices to supplant paper-based outcome measures, as well as other physician-rated instruments.
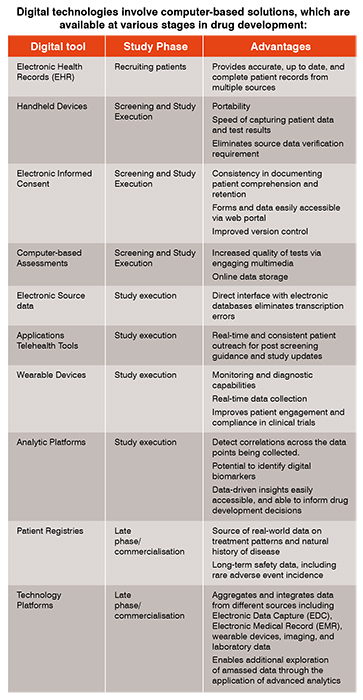
Wearables sensors have been used to a large extent as monitoring devices for heart rate, sleep activity, step count, blood pressure, oxygen saturation and temperature measurements. In neurodegenerative diseases like Parkinson’s disease, devices incorporating accelerometry and electromyography are capable of measuring motor activity, yielding critical objective assessment of disease severity and response to therapy. The sensors in these devices can be paired with other applications (such as those in mobile phones) to measure tremor, dyskinetic movements, gait and balance (Kubota 2016).
Other types of wearable devices can be used in a diagnostic or biomarker capacity, including assessments of walking speed in Multiple Sclerosis, seizure detection via wearable ECG devices, and measurement of perspiration in Cystic Fibrosis.
The adoption of sensitive and efficient instrument scan increase study power, thereby requiring fewer participants and accelerating study conduct.
Digital technology has transformed how companies approach clinical development by incorporating valuable insights from multiple sources of data. Data analytics can be used to inform virtually every facet of the clinical trial. Companies want more efficient ways to capture, aggregate and manage the right data. Analytic platforms provide a single source of operational study data that is used to guide decisions through visualisation tools. For example, study metricscan be analysed and communicated in a dynamic way to ensure key decision-makers understand them. These insights can also come from aggregated data over numerous studies in the same indication or therapeutic area.
Data analytics can also be adopted in the early stages of study conduct. In studies involving the central nervous system, the quality of psychometric rater data is optimised by periodic audio and video surveillance. Outliers are therefore easily identified, with the opportunity to correct issues earlier during study conduct. This algorithm is easily applied to other studies with similar assessments.
Virtual (or direct-to-patient) clinical trials are emerging as a valuable development tool, as these studies use technology for patient engagement, and for collection of safety and efficacy data, while eliminating the need for face-to-face clinic visits. They are of particular interest in orphan diseases, and in study populations with limited travel ability. Data collected in patient registries can offer insights into the natural history of disease, and outcome-based data may be linked to other data sources such as electronic medical claims data. As a consequence, registries enable transfer of real-world data sources into evidence that can improve health outcomes. Another advantage of patient registries is the ability to identify new biomarkers and clinical endpoints from the repository of data. This further stimulates new research on the causes, treatment, and outcomes of various conditions.
With use of DNA resources from EHR, the Myocardial Infarction Genetics Consortium Investigators (Stitziel2014) were able to make inferences about how the pharmacologic action of ezetimibe mimics the genetic inhibition of the Niemann–Pick C1-like 1 protein. These naturally occurring genetic mutations that confer a reduced risk of coronary artery disease were previously unknown prior to the completion of the prospective cohort studies using samples from 22,092 patients.
In 2012, a number of major pharmaceutical companies formed the nonprofit collaboration TransCelerate BioPharma in order to investigate how digital technology could be leveraged to improve clinical trial efficiency. Various initiatives have grown from this collaboration including an industry wide information sharing and clinical data standard to drive efficiency and consistency in data collection, and to promote interoperability and integration of EHR/EMR. The placebo and standard of care initiative is building a collection of anonymised patient data from the control arms of hundreds of clinical trials. Reuse of data from previous studies can reduce the number of patients required in a clinical trial via access to historical controls. This is of particular relevance in studies with rare disease populations, where use of placebo is infrequent, challenging, or both.
Genomic medicine involves the application of genetics to diagnose and treat disease. Early application of genomic medicine in drug development can expedite regulatory and payer approval by identifying those individuals most likely to benefit from a therapy. Personalised medicine involves “therapeutic products for which the label includes reference to specific biological markers, identified by diagnostic tools that help guide decisions and/or procedures for their use in individual patients” (PMC 2017). In 2017, there were a record number of FDA approvals of such personalised medicines, including approval of the first three gene therapies. The European Union regulatory framework for pharmaceuticals has outlined a number of tools to help companies develop personalised medicines. Japan is using its largest biobanks to collect genome data in support of clinical research on genomic and personalised medicine.
It is anticipated that in the near future, detailed individualised biological and physiological data using a combination of genomics, personalised electronic health records and wearables will drive efficiencies in the drug development process. Well planned virtual studies, using endpoints acceptable to key stakeholders (i.e., patients, physicians, payers, and regulators) are poised to accelerate clinical development in a cost-effective way.