The median investment to bring a new drug to market is estimated at US$ 985 million, which is expected to rise further in the current COVID-19 era. To improve efficiency of clinical trials, lower the costs, and enhance data quality—while ensuring patient access to new treatments—the drug development industry is pursuing new seamless approaches to clinical trial design. The innovative approach to contemporary clinical trial designs like integrated trials, basket trials, therapeutic repurposing during the trial etc. will not only optimise the cost and speed but will inspire both investigators and pharmaceutical companies for cutting-edge scientific discoveries.
Traditional drug development can be time-consuming and demands high investment. Recent advances in medicine with the availability of certain powerful tools are enabling researchers to understand the inner workings of human disease at the molecular level, therefore leading to increased demand and potential of discovering and developing innovative medicines. The field of clinical research is expanding tremendously, and the clinical development programmes are becoming more complex and costly, owing to factors such as increased regulatory scrutiny, the growing need to demonstrate safety, efficacy and value of drugs, conducting trials in defined patient subpopulations or patients with rare diseases and various ethical considerations. Further, various environmental, biological factors contribute to increasing incidence non communicable diseases and new emerging infectious diseases which often pose unprecedented challenges for the global heath community as well as the conventional clinical development paradigm.
The median cost of conducting a study from protocol approval to final clinical trial report can be US$3.4 million for Phase I trials involving patients, $8.6 million for Phase II trials and US$21.4 million for Phase III trials. Many new approaches for clinical trials are using novel drug development tools, such as biomarkers, to identify patients that may respond to a therapy. To improve the efficiency of clinical studies, a spectrum of expedited clinical trial designs are being developed which aim for more efficient trials associated with reduced development timeline and less resource requirement. Especially in the global pandemic, e.g. COVID- 19, we are increasingly facing the need for efficient designs and analyses of clinical trials. Innovative trial designs are providing the possibility for researchers to shorten trials, improve success rates and increase the efficiency of clinical research. The innovative approach to contemporary clinical trial designs like integrated trials, basket trials, therapeutic repurposing during the trial etc. will not only optimise the cost and speed but will inspire both investigators and pharmaceutical companies for cuttingedge scientific discoveries. One of the FDA’s initiatives has been to operationalise complex innovative trial designs. The FDA defines complex clinical trial designs as designs that are intended to advance and modernise drug development. Several generic drugs get approved on routine basis via this approach, reducing the huge costs and time associated with standard clinical RCTs .
Phase 0 clinical study
This phase includes exploratory clinical studies with less drug exposure than Phase I trials, to bridge the gap between preclinical studies and traditional clinical development and to facilitate decreased failure of drugs and improving the efficiency of drug candidates in later phases of clinical trials. Phase 0, also known as micro dosing studies, involve short processing time, early assessment of toxicity, efficacy and can provide the ‘go-or-no go’ decision making prior to a formal RCT. Phase 0 results guided with respect to required alterations for cell cycle-associated kinase inhibitor, AZD1775 for treatment of glioblastoma.
Seamless clinical trials
This approach integrates the 3-phaseprocessing of RCTs into a comprehensive clinical study without phase gaps. Phase I/II is designed to examine the most efficacious dose based upon some surrogate end-points such as Objective Response Rate (ORR), this dose is then continued into the confirmatory stage for further testing without a timing gap, to obtain more definitive end-points such as overall survival.
Programmed death 1-blocking antibody Keytruda achieved Accelerated Approval (AA) by the FDA based on similar approach. Inhaled indacaterol, a long-acting b2-agonist for treatment of chronic obstructive pulmonary disease, was clearly improved through a seamless (Phase II-III) clinical trial.
Master protocols
This approach allows evaluation of multiple treatments, target populations or both within a single protocol in a more efficient and ethical way. Master protocols are quickly emerging as a critical tool for evaluating potential promising COVID-19 therapies such as SOLIDARITY trial outlined by World Health Organization (WHO) involves evaluation of the benefits and risks for several preventive candidate SARS-CoV-2 vaccines.
Master protocols can be categorised into basket trials, umbrella trials or platform trials.
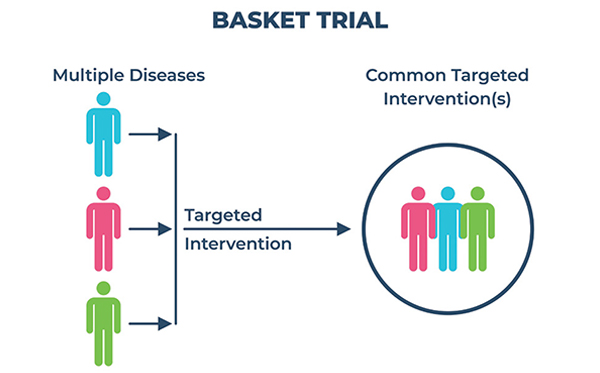
Basket clinical trials
The drug being tested is examined to reveal the therapeutic efficacy in wide spectrum of diseases simultaneously. Basket trial involves selection of therapeutically sensitive disease types and patient sub-populations, with a biomarker-based diagnosis followed by the disposal of tumour types with futile response and further evaluation of subjects with promising results. Larotrectinib an selective Tropomyosin Receptor Kinases (TRK) inhibitor is approved for wide spectrum of tumour types, reflecting a dramatic success of basket clinical study. Aberrant TRK activation is expressed in more than 20 distinct tumor types. This approach can address the unmet clinical needs of combating the rare neoplasms in oncology drug development.
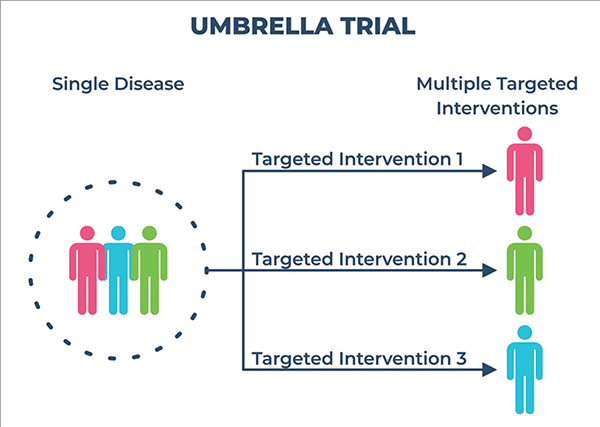
Umbrella trials
These trials evaluate multiple targeted therapies for a single disease in patients who have the same type of cancer but different gene mutations (changes) or biomarkers. Plasma MATCH is an umbrella trial that evaluated five different therapies for advanced breast cancer based on different predictive biomarkers.
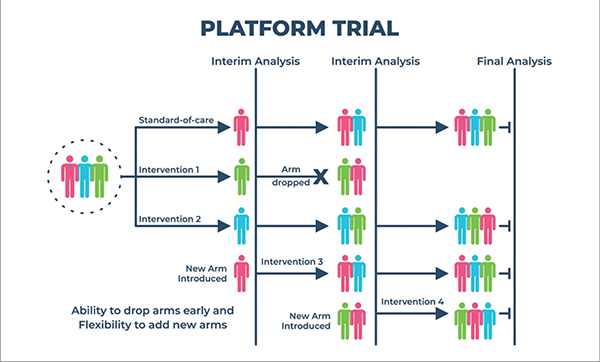
Platform trials
These trials include finding the best treatment for a disease by simultaneously investigating multiple treatments, using specialised statistical tools for allocating patients and analysing results which can involve dropping treatments for futility, declaring one or more treatments superior, or adding new treatments to be tested during the course of a trial. In 2013, the Innovative Medicines Initiative of the European Union (EU) announced platform trial for the prevention of Alzheimer disease to evaluate multiple treatments, from multiple sponsors, for persons at high risk for Alzheimer disease.
Therapeutic repurposing
Drug repurposing involves the identification of novel therapeutic indications for drugs which have previously obtained regulatory approval or been tested in clinical trials for other disease indications. This could involve a new formulation, delivery route or dosage of the drug and may potentially also involve a different drug target relative to the original indication. This study design allows re-purposing of drugs to an alternative indication, if the drug was facing lack of efficacy or safety issues in earlier studies and optimisation of trial to cover more therapeutic areas on the basis of the emerging evidence. The drug sildenafil was earlier designed for management of angina pectoris and was later redirected for treatment erectile dysfunction. In order to support therapy for COVID-19, the safety and tolerability of an anti-parasitic drug was tested on healthy volunteers.
Real-world evidence and real-time data
Real-world evidence (RWE) data involves the collection of demographics, family history, lifestyle, and genetics data which can be used to predict probabilities of diseases in the future. Real world evidence can be obtained from multiple non-interventional sources like patient registries, claims, observational studies, doctor visits, prescription data and connected devices. Clinical practice guidelines that have been using RWE-based insights include the National Comprehensive Cancer Network. Artificial intelligence is being utilised to for personalised medicine by identification of effect of comorbidities on therapy outcomes and subgroups in single disease. A recently published study that used RWE to compare cardiovascular outcomes between different therapies was the Cardiovascular Outcome Study of Linagliptin versus Glimepiride in Type 2 Diabetes (CAROLINA) trial.
Decentralised clinical trials
This is an approach to reduce costs and provide better access for patients by conducting trials directly with patients, also called ‘direct-to-participant’ trials. This includes the single centre overseen by a physician principal investigator, but otherwise has no clinical sites and no clinical investigators. Recruitment of subjects can be accomplished by using internet and interactive sessions via smartphones. COVID-19 has pivoted decentralised clinical trials, with e-consent, site less trials and digital tools used to connect with patients.
Adaptive clinical trials
Adaptive clinical trials are trials that allow for continual changes to the trial design based on data available. This helps in improving cost and efficiency, with a relatively high likelihood of success. An adaptive design allows prospectively planned modifications to the trial and/or statistical procedures of the trial after its initiation without declining its validity and integrity. Adaptive trials also aid in mitigating risks and delayed timelines. An example of adaptive trials is when trial dosing information is obtained from a single two-year combined Phase II/III, that may have taken a greater number of years and consecutive trials when traditional methods are followed. Adaptive trials also aid in lowering sample size, as the same patients may be used in multiple stages of the study. Such trials will also help in early termination of the study and reassessment of treatment measures and patient subgroups. A seamless Phase II/III was designed for 4vHPV vaccine according to which medium dose selected in Phase 2 was further used in Phase III confirmatory study and later own vaccine was licensed for the prevention of HPV 6/11/16/18 related cancer diseases.
Conclusion
Clinical trials play an essential role in evaluation the efficacy and safety of tested drugs for human use prior to marketing authorisation. The process of clinical trials is evolving continuously owing to the advancing research for pathophysiology and understanding of diseases on molecular level. However, due to increasing unmet need for development of drugs for various therapeutic areas, traditional clinical trials are facing challenges due to tedious procession, higher cost involvement, long duration of studies and stringent regulation. Unacceptable levels of attrition in the clinical stage of development are driving profound changes in the architecture, design, and analysis of clinical trials.31 There is an extensive need of development of innovative trial designs to increase the efficiency of clinical research. These trials provide a boost in clinical research by cutting on the cost and time factor and are contributing for development of many new chemical entities and drugs in view of the evolving era of personalised and evidence-based medicine.32 In future, more and more companies will embrace innovative clinical trial designs, thus improving the success rate of drug development.