Fuelled by the current COVID-19 pandemic, and the considerable volume of data generated during a clinical trial, Artificial intelligence (AI) has provided the much-needed impetus for transforming clinical trials into the virtual sphere for greater efficiency. From patient recruitment, protocol design, trial monitoring, to identifying the effect of blood thinners on virtual patients with irregular heartbeats, leveraging AI has helped life-saving drugs reach the market sooner.
Healthcare today faces extraordinary challenges posed by the COVID-19 pandemic along with a rise in chronic disease burden worldwide, an aging population, and the growth of the middle-class Asian population. According to the World Health Organization (WHO1), by 2020, an estimated three-quarters of all deaths globally would be due to chronic diseases. These stressors have helped facilitate innovations in conducting clinical trials in a bid to curb rising costs and reduce the time needed to conduct them. Simultaneously, there have been significant breakthroughs in science and technology, enabling intelligent clinical trial solutions.
A recent report by Researchand Markets stated that the global market for e-Clinical Trial technologies2, catalysed by the COVID-19 pandemic, is an estimated US$5.4 Billion(2020), with an expected rise to US$ 9.9 Billion by 2027. The U.S e-Clinical Trial Technologies market is at an estimated US$1.6 billion this year, while the market size in China is expected to be US$1.7 billion by the year 2027. Among regional markets, China will be one of the fastest-growing, followed by Australia, India, and South Korea, with an estimated Asia-Pacific market value at US$1.1 Billion by 2027.
Data provided by ClinicalTrials.gov shows that there was a reduction in the number of new trials between January and May 2020. Moreover, Michael Lauer from the US National Institutes of Health stated that nearly 80% of non-COVID trials3 were either stopped or interrupted. Investigative sites had to resort to ingenuity and flexibility during the subsequent period of recovery from June to July, with prior investment on the right technology aiding in risk mitigation. For instance, AI and machine learning (ML) platforms were leveraged by a global clinical research organisation to run six COVID-19 clinical trials using remote monitoring practices, while banking on vast experience and in-depth infectious disease expertise, that resulted in milestones reached ahead of time.
Furthermore, the advent of digital solutions in clinical trial management and conduct has improved transparency, with an onus on delivering better healthcare. Consumers or patients have access to a wide range of information, and, with this dissemination of information, there is increased expectancy. This has initiated a need for rethinking clinical trials to maximise benefits. There has been a significant shift towards embracing the incredible advantages of data analytics, along with digital models of engagement, to forge clinical trials that cater to the current demands.
Significant strides in incorporating digital health solutions began a few years ago, more as experimental solutions or as support for certain sections of clinical trials. Such investments have paved the way for hybrid clinical trials that are guiding forces for successful and efficiently run clinical trials.
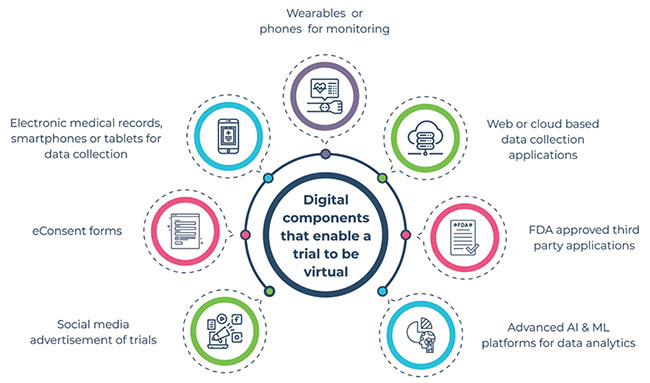
A virtual clinical trial harnesses the power of technology to improve patient recruitment, retention, collection of data, and analysis. They support efficient trials as they tap into digital technologies, like apps, monitoring devices, and online social engagement platforms to conduct each stage of the clinical trial. This includes enhanced support for recruitment, informed consent, patient counselling, measuring clinical endpoints, and in determining adverse reactions.
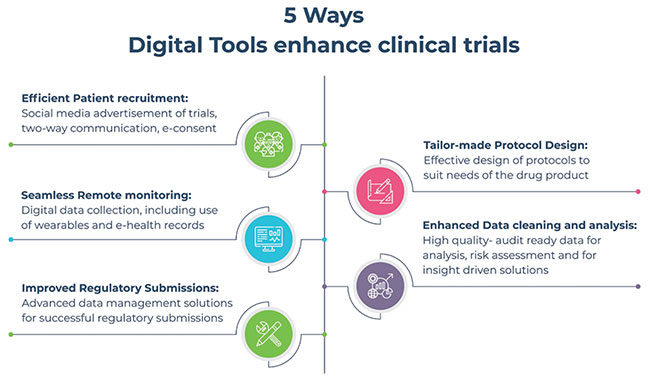
1) Patient recruitment: Clinical protocol development is the first step in a clinical trial. With the multitude of data sources, like imaging health, genomics, and patient-reported outcomes, there has been a shift in trial protocols to meet regulatory requirements. Multiple reasons could hinder patient recruitment, including involving populations that are hard to reach, people with disabilities who cannot come to the trial centres, lack of awareness about the trials, and the financial burden on the study population if there is a need for frequent trips to the research centres.
Leveraging digital methods to facilitate recruitment has been in use since even before the current dictates of the pandemic. Encouragingly, such trends have been the reason for optimism, with clinical trial announcements popular over social media channels. Patients don’t have to travel to sites to sign up for the study; instead, they could send in e-consent forms. Technology reaches patients who would be most suitable for the study, ensuring that they participated with minimal travel to the site, significantly increasing patient participation and retention during clinical research studies. Such
innovative solutions ensured that the first patient for a safety and efficacy trial for a COVID-19 drug product was recruited within 26 days of the study being commissioned.
To ensure seamless collaboration, detailed and uniform training for effective management and use of digital tools for protocol adherence, recruitment, sample management, PD/Issue Handling, data entry and cleaning requirements, good source documentation, regulations etc., need to be provided.
2) Digital health data collection: After patients are recruited for a study, it is important to collect data during the clinical research study process. There are multiple ways to collect this data using digital tools. Demographic and medical health data, patient activity, and physiological parameters, patient-reported outcomes, along with images, can now be collected using electronic medical records, smartphones, or tablets.
There has been a dramatic shift towards digital monitoring and biomarkers. This refers to objective measures of behavioural, pathologic, physiologic, anatomic, social, and patient self-assessment, which can be obtained remotely using digital technology and used as a means of evaluation. Examples of such technology include wearable activity trackers or phones with sensors to detect cardiogenic chest wall vibrations as a means of identifying heart failure or heart rhythm. Other examples include sensors to detect sweat for glucose, lactate, electrolytes, or sensors placed in braces to determine structural health markers for knee joint injury. Statistical analysis may be used to identify the effect of blood thinners on virtual patients with irregular heartbeats.
Digital technologies have also helped in collating data that couldn’t be collected through traditional means before. A case in point is the use of unique applications available in smartphones. Patients need to perform a set of tasks indicated in these apps, which is used to detect signs of Parkinson’s.
Regulatory authorities have also been closely monitoring such advances in technology and data collection. The Food and Drug Administration (FDA) approved the use of the Apple watch in detecting heart rhythm abnormalities, like in atrial fibrillation, that use optical sensors for photoplethysmography and electrodes for electrocardiography.
e-Source may be used as the primary source of patient data capture, except for informed consent form and laboratory data. The data from e-source may be synchronised with electronic data capture, so there is reduced data input required from sites.
3) Safety monitoring in virtual trials: The fast pace of digital technology movement into routine processes in a clinical trial has helped in significantly improving efficiency, reducing time and cost for sponsors. New tools are now being paired with traditional biomarker assessments to enhance safety and validation.
A considerable advantage of using digital tools in safety monitoring is that there is continuous data collection that can be used to detect infrequent events or even to identify situations that may not occur during a study visit. In a bid to better monitor clinical trials, artificial intelligence, and machine learningpowered digital platforms have been in use for many years to identify potential risks. Such platforms facilitate near realtime data insights that promote faster detection of events and reporting, which has a considerable impact on clinical trial timelines and cost.
One of the critical factors in incorporating digital tools in virtual trials is meeting safety and regulatory standards.
4) Data Security: To develop intelligent clinical trials that do not require constant human monitoring but are dependent on advancements in technology, certain challenges need to be addressed. There is an increased need to tighten data security during collection, transmission, and analysis. There is a heightened risk of breach of data regarding the location that could affect the study participants.
To overcome this, the FDA has put forward specific guidelines, like conveying information collected by the digital tools to all stakeholders. Medical device certification has been developed as a measure to control data breach.
There is a need to understand the regulations that govern the use of data within specific geographical regions and the changes in rules that may exist in other areas. There is reduced dependency on physical sites in virtual trials, and patients may be enrolled from different cities or even countries. The rules that govern data collection, use, and inclusion in studies vary from one state to another and from one country to another. It is important to stay abreast of the latest in the field to ensure successful clinical research study outcomes.
The use of wearables and smartphones in data collection provides a continuous stream of information that is transferred to study investigators using web-based applications. This type of data transfer may be at risk of a security breach. The FDA is developing a risk-based approach in better regulating such third-party applications. Wearables provided by Apple and Fitbit have the necessary certificate as they follow the FDA's guidelines.
There have been warnings issued by the FDA against the use of certain insulin pumps and an implantable cardiac device as there were vulnerabilities in the pump that could result in tampering of the device by unauthorised people. The use of blockchain technology may be one potential solution to ensure data privacy and security. Though such vulnerabilities exist outside the clinical trial sphere, it is essential to use the right technology to ensure data security.
Virtual trials have a compelling advantage over traditional clinical trials when the technology is developed following guidelines.
5) Data analytics: Flexible, extensible, and scalable clinical trials can be carried out only with the support of effective data analysis. For example, an AI and ML powered platform enables study investigators to connect remotely and access data from clinical trials in near real-time. Such emerging technology helps automate processes and mapping data, with advanced analytic methods applied to manage multiple facets of clinical trials.
Such artificial intelligence and machine learning platforms help augment data extraction and in computational phenotyping that enhance efforts towards successful clinical trial outcomes.
6) Optimising trial methods: There are multiple ways in which virtual resources support clinical trials optimisation. A method of intervention optimisation, called micro-randomised trials, involves identifying factors like dose and timing that can be managed better using reminders. Such engagement strategies are best suited for patient recruitment, enrolling and retention. The personalisation of the clinical trial process helps in enhanced patient participation in clinical trials.
Optimisation of clinical trials can extend to other aspects of the trial as well. In time, it will help in building robust systems that greatly enhance the conduct of biomedical research.
The traditional clinical trials or the in-site system may not be effective during the current unprecedented times, however, the benefits of virtual clinical trials extend beyond the purview of the pandemic. There has been an increased dependency on innovative and intelligent solutions in managing clinical trials over the past two decades.
Societal interaction that has largely been based on face to face interaction is now slowly moving to the virtual space. Though this transition has been slow in the clinical trial sphere, many tools are now being embraced to re-engineer clinical research. Robust methods to track the progress of clinical trials using AI powered platforms, or remote monitoring using e-Source, with intelligent trial management using the trend output from such digital platforms have significantly elevated the conduct of clinical trials.
Large volumes of data from clinical trials can now be cleaned from the time of the first person enrolled in the study till the end of the study. Digital tools can also be used for periodic medical data review and to streamline clinical trials, with faster decisions taken on the drug development process.
Improved efficiencies throughout the clinical trial process will aid in reducing time and costs. The use of the right digital health solutions allowed rapid site activation within 20 days, faster recruitment of patients and comprehensive trial oversight for a safety and efficacy drug trial on moderate to severe COVID-19 patients. The innumerable benefits when using digital tools to support clinical research reinforces the need to incorporate new forms of technology which act as critical enablers to achieve better coordination and in simplifying processes between the various stakeholders and ecosystems in a clinical trial.
Despite the current COVID-19 times of difficulty and uncertainty, clinical trial conduct experienced a ray of hope demonstrating real feasibility of virtual clinical trials. Virtual clinical trials have demonstrated the ability to improve access, reduce participation burdens, and enable the collection of robust and secure data, among other benefits with the support of digital tools. To stay at the forefront of innovation, it is imperative that organisations understand critical healthcare needs, evaluate their capabilities and make necessary plans to deliver virtual clinical trials to patients.
Reference:
1. https://www.who.int/nutrition/topics/2_background/en/
2. https://www.businesswire.com/news/home/20200908005697/en/Global-e-Clinical-Trial-Technologies-Market-Trajectory-Analytics-2012-2019-2020-2027---ResearchAndMarkets.com
3. https://www.thelancet.com/journals/lancet/article/PIIS0140-6736(20)31787-6/fulltext