The global financial crisis is impacting almost all industries—including pharmaceuticals. Minimising cost is a growing focus in the industry. In Asia, the impact comes in two forms. On one hand, pharma companies are motivated to scale their development operations to cover low cost countries. On the other hand, there is increasing scrutiny on development costs in Japan. Indeed, it has become a pressing issue for the heads of R&D in Japanese subsidiaries of global pharma companies. Many say that clinical development costs in Japan are at least twice as much as those in the US. Meanwhile, PRTM survey data shows that even the best companies run at about a 30 per cent premium in Japan.
Executives have three “levers” available to them to manage clinical development operational costs such as:
1) Taking advantage of low cost countries in Asia and other areas around the world,
2) Outsourcing specific operational activities, and
3) Implementing “lean” clinical development practices.
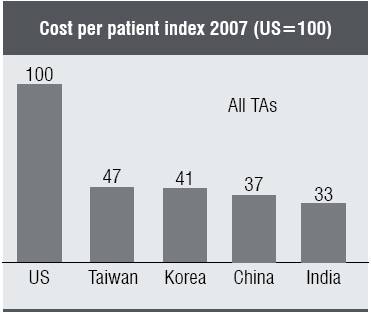
With deep experience in the pharmaceutical development, PRTM understands that none of these levers are easy to use. However, successful companies are developing the necessary management discipline to effectively utilise these levers.
1. Off-shoring clinical development operations—to low cost countries in Asia and elsewhere in the world—will soon become a standard tool in defining a global clinical development strategy. Plans now need to take care of many requirements such as diverging regulatory requirements from a wide variety of countries—as well as total program cost. Cost data shows that cost per patient runs as much as 60 per cent lower in Asia than that in the US (2007 data). Meanwhile, Japan’s regulatory agency, MHLW, has become open to using global and Asian patient data for filing. However, this requires the proper infrastructure and processes for success. At this time, most global pharma organisations are not organised properly in Asia.
2. Outsourcing clinical operations is also an increasingly common practice—including in Japan. This often provides organisations with increased resource flexibility and can help reduce overall cost. In spite of this goal, operational managers in pharma companies often complain that using CROs results in higher costs than using employees. Some global companies have even decided to reduce outsourcing. On the other hand, successful pharma companies are able to realise reductions of as much as 20 per cent. Successful companies have established specific management practices to effectively work with outsourcing partners.
3. The third lever is to establish lean clinical development practices. This involves the concept of solving the fundamental issue of clinical operations—namely inefficient site-management. Leading pharma companies are applying global best practices such as preferred site selection, remote monitoring, or sampling Source Data Verification (SDV). Often, companies try to apply these practices, but give up midway. However, successful implementations of global best practices have resulted in productivity improvements of around 30 per cent.
Pharmaceutical industry executives have various levers available for optimising development operational costs. The core of competition is developing a disciplined management team which effectively creates a sense of organisational urgency, understands the nature of the effort, and skillfully operates the available levers to achieve cost targets. With the right approach, companies can achieve significant improvements.