Patients form the centerpieces in determining the validity, efficacy and success of clinical trials. With identification, screening and enrolment of the patients tricky enough, the retention of enrolled patients proves to be an insurmountable hurdle as trends state that nearly 85 per cent of the clinical trials are unable to retain patients till the end of the trial. Various techniques have been discussed and employed for attaining a healthier statistic including incorporation of patient inputs in designing the trial. Innovation today has helped boost this further in the forms of utilisation of technology in effective patient engagement.
Introducing a new drug or treatment regimen is a laborious task which is filtered at various levels before it reaches the general populace. One of the final filters is Clinical trials which establishes the efficacy and safety of the regimen; improves patient outcomes and maximises the success of the new entity in the later stages. Although testing of a regimen prior to the efficacy and safety establishment poses a risk to the participants voluntarily enrolled in the trial, the clinical trials today are under strict regulations and take sufficient measures to minimise the risk. Additionally, the patients enrolled are benefitted by being administered a treatment not only before it is publicly available but also with personalised attention, care and frequent monitoring.
Despite the numerous benefits, enrolment of patients in clinical trials often proves a bottleneck for the researchers. Enrolment of patients for the process of clinical trials is a long and multistep screening process critical for achieving the objectives for a clinical trial. The first hurdle is identifying the right patients that fit your requirements. Once identified, the patients are screened for suitability, educated and informed about the trial and finally randomised. At each step of the process, the pipeline of the patients leaks and tapers to give a final randomised patient list which are enrolled. Figure 1
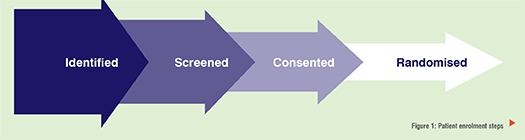
Enrolling patients successfully is only half the battle won. Since participation in a trial is a voluntary activity, it becomes strenuous to ensure the retention of participants till the complete schedule of the generally lengthy clinical trials. As research shows, only 7 per cent participants of the initially identified participants successfully complete the trial. The validity of these trials depends largely on the discipline of the patients enrolled and their adherence for the regimen.
The foremost requirement for improving patient enrolment and retention is to understand the roadblocks and hurdles in the process. Considering patients an integral part of the process, it is important to understand the concerns and expectations of the patients with respect to all the stakeholders while designing a clinical trial. Figure 2 maps the clinical trial process and the problem areas encountered in each phase which generally leads to low enrolment and poor retention. Figure 2
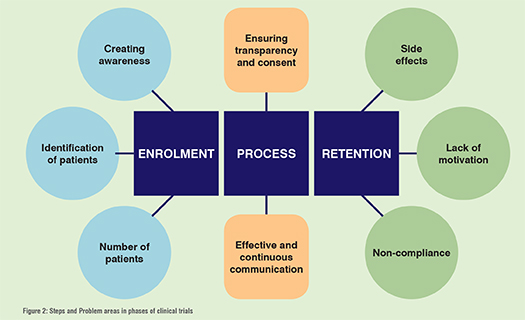
Once the barriers are identified and understood, the improvement strategy in each stage can be chalked out and implemented. To maximise the retention of participants through the length of the trial, the regimen must ensure transparency covering the length of the trial and beyond.
Various studies have been carried out to determine these factors and optimise the trials. Despite the palliative steps undertaken to reduce the ‘leakage’ of the participants through the trial, it remains an uphill task. With the advent of technology which is so closely associated and accessible to the participants, an enormous potential emerges for their effective engagement. It is important to tap this potential for not only improving the enrolment and retention but also enhancing patient outcomes.
As the industry is getting disrupted by introduction of digitisation and artificial intelligence at every step of the drug development process, clinical trials have not been untouched. Digitisation has wriggled its way to the minutia of the process and opened an entirely new horizon of possibilities. Coupled with the current physical ways of handling the task, it paves way for a novel ‘Phygital’ strategy which provides end-to-end interventions and decongests the common bottlenecks encountered. Fig. 3
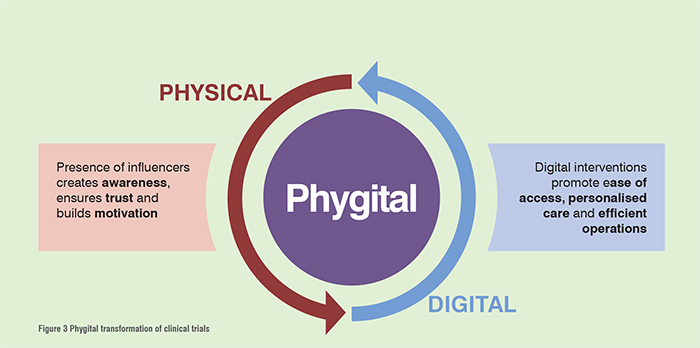
Let us have a look at the common bottlenecks encountered during clinical trials and how Phygital transformation helps them.
i. Creating awareness: The skepticism of patients to enroll in a clinical trial stems from the lack of trust on the reliability of the trial. It is significantly improved with the presence of an expert whom they perceive to be dependable such as a doctor or other healthcare professionals. Engaging influential figures for creating awareness about clinical trials can help to motivate the patients. Influencers from the digital sphere are increasingly getting engaged with clinical trials and promote such trials to rope in participants.
ii. Identification of patients in qualitative and quantitative spectrum: A clinical trial is designed to achieve a set of objectives which can be attained through data obtained from a specific group of individuals. The parameters for screening these individuals often involve a complex web of requirements including but not limited to disease conditions, ethnicity, genetic profile, gender and age to develop a robust trial. Traditionally, each prospective participant was identified and screened to determine their suitability. With digitisation and AI, apps have been developed that take the profiles of the participants through a network of algorithms and find the right match suitable for you! Not just qualitatively, the intervention greatly reduces the time frame required thus increasing the number of patients identified in the given time period.
iii. Ensuring transparency of the trial: Misunderstood expectations and incomplete understanding of the particulars of the trial prove to be a hindrance in the smooth running of a trial. Recent technological advancements allow creation of interactive web experiences that provide a walk-through of the process and empower the participants. Such a platform is helpful not just at the beginning of the trial but also assists, guides and counsels them at every step.
iv. Communication: Through the course of the trial, patients often find themselves demotivated due to lack of any significant improvement in their condition or even the lack of appreciation for their effort. Creating milestones and providing a regular feedback recognising and applauding their effort can go a long way in motivating the patient. Automation of a personalised feedback mechanism presents an opportunity for regular positive communication highlighting the significance of the contribution the participant is making towards better healthcare.
v. Minimising risk of side effects: The biggest hitch in patients enrolling for clinical trials is the possibility of encountering side effects. Often, identification and reporting of side effects are delayed. These can be curbed by continuous monitoring which is at our disposal by the benefit of the internet and interactive ‘chat-bots’. This additional dimension makes the process of trials conducive for the patients since assistance is just a click away.
vi. Maintaining compliance: A major reason that proves a hurdle for successful data collection is that compliance for the regimen is not maintained at the patients’ end due to various reasons such as incomplete knowledge or simply forgetting the schedule for visits and dosage. An easily accessible digital planner and reminder which provides consistent updates of the schedule can markedly improve the compliance and thus the validity of the trial. It ensures the collection of right information from the right patient at the right time!
Way towards qualitative and cost-effective clinical trials
Nearly 85 per cent of the clinical trials are unable to retain the enrolled patients till the entire cycle of clinical trial. They lose about 15-40 per cent of the participants on an average due to various reasons. This leads to discrepancies in the data and sometimes even an inconclusive trial result. The reasons for drop-outs are varied and are generally difficult to control through a broad scale of measures. Retention calls for a more personalised care and constant mentorship for each participant. Having a personal mentor which patients can perceive to be dependable, capable and trustworthy greatly motivates them to adhere to the requirements. Such personalisation for a large population becomes manageable through digital platforms and AI along with the physical presence of a trustworthy influencer such as a doctor.
On the whole, incorporation of Neuro-linguistic programming and machine learning can provide a holistic solution wherein the key lies in execution and flexibility of the platform.
Optimised clinical trials can prove highly cost-effective in the areas of recruitment and retention of the patients as demonstrated by a pioneering app in this field — a 92 per cent reduction in resource requirement for conducting the study. With higher enrolment, clinical trials would lead to introduction of better and advanced care for the world and help us combat diseases better in a reduced time frame.