Consolidating and integrating data across systems and relationships can help manage some of the challenges associated with supplying investigational and comparator drugs for clinical trials.
Ensuring the efficiency and validity of clinical trials requires that several factors come together, not the least of which is the investigational material. The supply chain underlying a clinical trial is a complex entity, with product passing through numerous hands before reaching the clinic and, ultimately, the patient.
Understanding the challenges
Clinical trial supply managers face several challenges in tracking drugs through this journey, including finding ways of managing supply data. For instance, to make sure that all necessary tracking data is captured, trials have information systems in place at every point along the supply chain, such as forecasting, inventory management, labelling, and distribution (Figure 1). These systems are not necessarily interoperable; there are no industry standards for clinical trial supply chain management akin to Clinical Data Interchange Standards Consortium (CDISC) standards which are widely used for Electronic Data Capture (EDC). Thus, to centralise such information, sponsors generally have to write proprietary code that allows disparate systems to exchange data.
Keeping in mind that we use the term “information systems” as an overall descriptor, one should not necessarily assume that all of these systems are automated or electronic. Many trials still rely on hand-entered data in spreadsheets or even paper-based systems. These practices can be particularly problematic in the event of a regulatory audit, as they force sponsors—who are ultimately responsible for all data from all aspects of a trial, including supply management—to spend significant time and resources reconciling data.
The practice of outsourcing complicates the picture. A recent survey in Contract Pharma ( Roth G., 2008, 4th Annual Outsourcing Survey, Contract Pharma, Vol. 10. Issue 4.) revealed that of 175 biotechnology and pharmaceutical companies, 48 per cent engaged six or more preferred outsourcing vendors in their clinical trials. The path that a drug may take through the series of vendors can differ from protocol to protocol. As noted above, the trial sponsor is ultimately responsible for gathering all relevant information generated by the trial, including data from outsourcing vendors.
Trial design is also evolving, with adaptive trials becoming more prevalent. The dosage of an investigational drug, or the number of trial participants, can change dramatically following an adaptive trial’s interim analysis, which can significantly impact supply forecasting and management. Also, trials are crossing international borders, reaching out to include patients in multiple countries so as to achieve greater power. Thus, supply plans (and as a result, the infrastructure for information management) must account for the regulations of each participating country, including packaging and labelling rules, and ensure that an appropriate amount of investigational drug and comparator drug (which may differ depending on which drugs are approved in a particular market, adding additional complexity) reaches the patients in each country.
Bringing data together
The software industry has responded to these challenges with a number of IT solutions, some based on adaptations of data management systems developed for commercial manufacturing and supply. Such solutions, however, do not meet the particular environment of the clinical trial, where lot sizes are small, packaging and labelling requirements are unique to the protocol, and doses must be blinded to ensure the integrity of the trial’s clinical / biological data and conclusions. There are a variety of other solutions employed in the supply chain including integrated, “purpose-built” systems, more evolved forecasting and simulation tools and data warehousing and business intelligence tools.
Of these solutions, those based on data warehousing and business intelligence are starting to play a more critical role. Before going into these, some definitions are in order. Briefly, a data warehouse is a repository of an organisation’s electronically stored data. Business intelligence tools are applications designed to report, analyse, and present data, providing the analytical and presentation (dashboard) functions that allows data to be used in decision making. A third concept that should be included in this discussion is the data mart, an organisational data subset (a room within the warehouse, if you will) that is usually oriented toward a specific task, purpose, or data subject.
The idealised supply chain management scenario centres on the data mart, containing all that subsets of organisational data (from the data warehouse) having to do with clinical trial inventory and supply. The data mart serves as a data repository, as an engine for consolidating and transforming / adapting data from vendors or suppliers to common formats, and as a means of receiving, storing, and routing inventory requests. Information from all of the varied supply management systems noted earlier in this article would pass through and be stored in the data mart. Business intelligence tools serve as means to access (ideally, via Web-based portals), share, query, and act upon data within the mart so as to respond to the needs of the trial sponsor and trial locations (Figure 2).
There are numerous benefits to solutions that combine data marts with business intelligence tools. For instance, such a combination of tools allows all members of the trial, including the clinical team, to access supply data and adjust their own planning accordingly. Business intelligence tools allow for improved inventory decision making and flexibility to respond to changes in protocol (a boon for supply chain managers taking part in adaptive trials). By consolidating and presenting all supply-related data in one location, the data mart-business intelligence combination allows for more efficient reporting of inventory at the end of a study. Lastly, business intelligence tools can serve as a dashboard for senior management, facilitating a top-down view of product supply and distribution and the Key Performance Indicators (KPIs) that are used to measure the health of the organisation’s internal and external supply chain operations.
Considerations when designing a solution
Implementation of such solutions is not without its own challenges. For instance, as noted above, the industry has not agreed upon standards for formatting, storing, and exchanging supply chain data. For now, most organisations overcome this challenge by creating proprietary transformation and exchange tools that bridge disparate systems. This is not, however, an ideal solution, as the process for developing, validating, deploying, and maintaining such bridge tools is onerous and time consuming. Such solutions also do not provide adequate flexibility to accommodate various system upgrades or easy replacement of one or more of the component systems in the supply chain. For this reason alone, a concerted industry-wide effort towards development of standards would bring new efficiency to supply chain management, just as it has for data capture and trial management.
If a combination data mart-business intelligence system were to be used in GMP-related decision making or study reporting, it would have to undergo system verification. This is a time-consuming process, but one that would give assurance of the reliability and fidelity of the storage solution and accuracy of the analyses generated using the business intelligence component—an important consideration when looking forward to regulatory submission.
Data contained within a data mart is only useful if it can be queried and accessed in ways that fit an organisation’s reporting and analysis needs. Thus, systems built on this combined model must be constructed with intuitive and flexible reporting in mind. The data mart must be understandable in order to allow the inclusion of ad hoc reporting. KPIs, which can be of great help in pointing out areas for improvement that would increase overall organisational efficiency, can be difficult to extract from supply chain and inventory data unless the system is built with the foresight to allow for measurement of the proper metrics.
Information systems have evolved such that decision making in near real-time is possible. But how real-time will a data mart system be? Or need to be? Because of the limited availability of industry experience with such solutions, the first question is difficult to answer. The second can only be addressed by a thorough examination of trial protocol’s information needs, with particular consideration of decision timeframes and the temporal resolution needed to support them.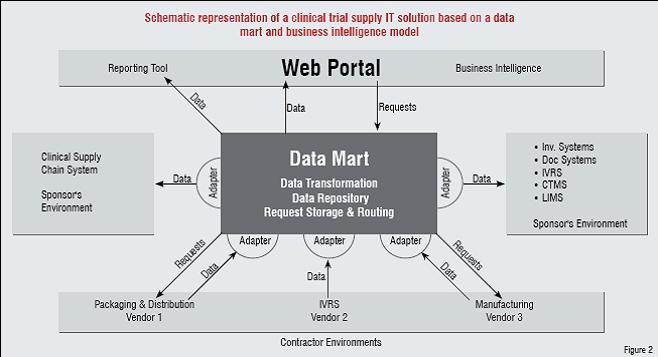
The ten-year view
Whether this kind of data consolidation and storage can in practice provide the benefits noted above remains to be seen, as it has not yet been deployed in a widespread fashion. However, this model feeds directly into a vision of how the clinical trial supply chain would look 10 years from now. In this forward-looking scenario:
• Manufacturing and packaging processes will be increasingly automated, if not entirely
• A standardised data dictionary will be applied to all clinical supply services
• The hosted “software-as-a-service” model will come to predominate as a means of controlling costs, thereby necessitating interoperability
• Label information will increasingly be published electronically, rather than in print
• Real-time patient enrollment data will be utilised to influence strategies and workflows for packaging and
labelling
• Planning, simulation and measurement capabilities will become more sophisticated
• Integration of sponsor and vendor data systems will achieve seamlessness.
This kind of automated, standards-driven paradigm of trial supply management will bring the same efficiency and integration now being seen in trial data collection. The development and adoption of information system models like data mart-business intelligence will accelerate the evolution of this paradigm. At the same time, it will offer significant advantages in trial efficiency now, enabling companies to more rapidly enroll and execute clinical trials and reduce the time to market for new innovative products.