IT can play a key role in cutting down the costs of production and helping biopharmaceutical companies maintain regulatory compliance. IT service management software can be an essential element in
As the complexity of the biotechnology industry increased, pharmaceutical companies have struggled to ensure that their IT organisations keep pace with the latest hardware and software applications. Yet, the need to maintain regulatory compliance while ensuring that an organisation’s IT infrastructure meet its ever-changing business requirements, is a significant challenge that industry faces today. Biopharmaceutical companies must deal with the regulatory pressures of cost-effectively maintaining a qualified IT infrastructure with validated business applications, while dealing with the business demands of managing and controlling an IT infrastructure through effective IT Service Management (for example: change control or incident management). IT service management software can help companies cost-effectively meet their needs in both of these arenas.
ITIL – A framework for compliant IT service management
Regulatory compliance is one of the critical elements in the pharmaceutical industry that drives a significant portion of IT spending. Consequently, proper IT service management plays a significant role in reducing company risk by maintaining compliance in a cost-effective fashion. One of the most effective methods of achieving IT compliance is by adopting a process management framework such as Information Technology Infrastructure Library (ITIL®). The ITIL framework is a proven and accepted model for regulatory compliance, especially since process requirements for a mature ITIL model are in line with FDA requirements.
By using ITIL as a framework and incorporating common enhancements to ensure FDA regulatory compliance, such as approval levels, record controls, and integrating risk management techniques, a pharmaceutical company’s IT department can cost-effectively maintain compliance. Adopting ITIL allows companies to measure, monitor and analyse processes that enable implementation, management and continuous improvement of their IT services and underlying infrastructure. ITIL also enables companies to better incorporate risk analysis and management in their procedures.
Many pharmaceutical companies are moving towards, or have adopted an ITIL-based approach to IT service management. However, due to the documentation required to demonstrate regulatory compliance, most pharma IT processes are paper-based and not easily accessible throughout the enterprise. As a result, there is a significant opportunity to reduce the cost of managing IT infrastructures while increasing the level of regulatory compliance. This can be accomplished by automating ITIL processes using IT service management software.
Driving consistent processes through automation
From the perspective of managing IT delivery, IT service management software can help ensure compliance through the integration of all IT processes. These software tools allow for the continuous flow and visibility of information. IT Service Management can provide a single, enterprise-wide platform for incident management, problem management, change and configuration management, release management and service level management.
IT Service Management will also reduce the cost of maintaining IT infrastructure in a compliant state by allowing for optimisation and stabilisation of existing processes and electronic management (authorisation and approval) of IT infrastructure management process and procedures. This will reduce the cost of IT Infrastructure compliance and qualification by eliminating much of the paper-based documentation and process / procedure approvals.
For example, in the realm of change control, IT management software with ITIL-based procedures can help streamline processes and approvals while reducing the risk of implementing unauthorised changes. Pharma companies can benefit from software that allows them to quickly implement standard processes throughout an enterprise, with proper approval controls and mechanisms, while maintaining 21 CFR compliant digital signatures, audit trails etc. The software should also be able to track all changes, allowing IT managers to look at the overall processes from a workflow perspective where they can evaluate and control the impact of system-wide changes. With IT Service Management, IT managers can easily manage all related tasks and actions in a change process, tracking action items through completion. From a compliance perspective, IT (and compliance) managers can more easily capture, and manage, exceptions and deviations.
Accelerating the validation process through automation
Another area where software can drive compliance as well as help to better manage the IT infrastructure is in the area of computer software validation. Processes and tools must be developed and implemented to maintain the software and hardware involved in FDA-regulated processes in a validated state. ITIL and IT automation software tools in combination with some specific enhancements, can help biopharmaceutical companies automate the processes around software validation in order to ensure that they are able to take advantage of the latest IT technologies, while lowering costs and risk and maintaining compliance with regulatory requirements for IT qualification and validation.
It should be recognised that computer software validation does make good business sense. It is less expensive to validate proper system functionality up front than to deal with inconsistent performance later. However, effective computer software validation must be an integral part of IT operations. The need to maintain applications in a validated state has encouraged some negative behaviours, such as:
• Reluctance to perform patches or upgrades because of need to revalidate applications
• Manual validation processes often result in out-of-date IT environments that can be very difficult and costly to maintain.
Leveraging test management and automation software will allow biopharma companies to improve their validation processes and reduce compliance risk through the automation of their current paper-based processes. These benefits include time and cost reduction, increased consistency and quality, repeatability and enhanced agility, and automatic report generation. The end result will enable companies to deploy application patches and upgrades more frequently keeping IT infrastructures technologically current and compliant. This can be accomplished by incorporating automated functional testing, automated performance testing, test management and monitoring managed through software automation tools into the process.
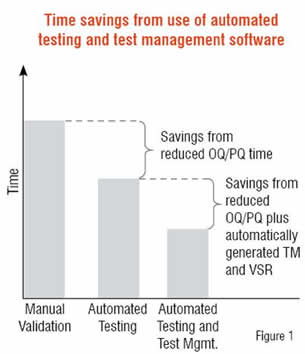
IT infrastructure software accelerates the validation effort by helping to automate elements of the validation process particularly Operational Qualification (OQ), Performance Qualification (PQ), traceability matrix and the summary report. The role of OQ and PQ in software validation is to assess the key metric of whether or not applications function according to their intended use. Regression testing helps assure that the functionality and performance of applications continue to meet expectations over time as changes occur to the system. However, manually maintaining a regression suite is costly and labour-intensive, and they must be re-run after patches or major software updates. These software tools reduce the amount of time devoted to OQ and PQ by automating and accelerating regression testing to dramatically reduce execution times while providing fixed and repeatable sets of tests. The result is that automated testing will reduce time, and costs in the areas of OQ, PQ, generating a Traceability Matrix and Validation Summary Report.
These tools also allow organisations to better incorporate a risk-based approach by allowing them to look at their integrated applications and focus on areas of the application that are critical to human safety. For example, should a company identify, through their risk-based analysis, an application that has a high-risk profile for drug safety; they can use them, in conjunction with a risk-based approach, to perform more rigorous testing on those application areas of highest risk.
Once a company completes its validation and automation, thereby improving its efficiencies and reducing the cost of initial testing, they can be further used to perform periodic testing. Good validation SOPs and IT processes also call for regulated companies to periodically test their IT environments. This is a perfect situation where biopharmaceutical companies can use automated testing tools for scheduling and conducting the required testing and save the results for later review and action. The use of these tools will allow an organisation to quickly and cheaply verify that their environment is in a validated state. Thus, management of an IT infrastructure becomes much more agile when companies can increase their testing to a more frequent basis through automation. The result is better assurance of a validated state and preparedness for validation audits as well as a better understanding of the operational status of their IT environment.
IT management and compliance through software automation
IT infrastructure software management tools can be a key weapon in the arsenal of pharmaceutical companies to help ensure both compliance and cost-effective management of their IT infrastructures. Many companies are discovering that deploying software tools for incident, problem, change and configuration, release and service level management as well as test management can actually help reduce compliance uncertainty and greatly streamline operations.
Properly deployed IT management tools and automation technology actually lowers compliance risk by enforcing standard policies and procedures to ensure a stable IT infrastructure. Coupled with ITIL practices these tools will allow organisations to evaluate and analyse their processes and procedures in terms of best-IT practices and their risk management profile. Validation processes are more efficient and effective with automation testing technology, which helps to ensure a thorough testing of validated IT environments. Automated tests reduce the time required for complex testing as well as ensuring their repeatability and consistency. The result is that organisations are able to better maintain an effective and available software environment that is current with the latest patches and upgrades.
Any life science organisation that is interested in the cost-effective, compliant management of its IT infrastructure can use IT Service Management software to help implement its ITIL service delivery strategies to positively impact the business in the highly- regulated environment of the pharmaceutical industry.