Nobody challenges the fact that Artificial Intelligence (AI), Internet of Things (IoT), Real World Data (RWD), and Blockchain will fundamentally impact drug development. While there are many emerging solutions, there is limited experience in applying these solutions in the real-world. In this paper we focus on how IoT and AI technologies can be integrated into a patient-centric digital platform and practically applied to enable new approaches to trial execution, while opening the way to a new generation of products.
2017 was the year where pharma realised that Big Data could dramatically increase the value of data produced during drug development or coming from Electronic Medical Records (EMRs) and medical devices. In 2018 it became clear that AI technologies could unlock much of the value of Big Data; 2019 is the year where we have everything in our hands to leverage Big Data, IoT and AI to deploy solutions that fundamentally affect the full drug development lifecycle, reverse the continuous growth of drug development timelines (25 per cent higher in 2018 versus 2012, reaching a startling 12 years on average) and bring new therapies to the market sooner and smoother.
The IoT refers to the ever-growing network of physical objects — beyond computers, smartphones and tablets — that contain electronics, software, and connectivity allowing these things to connect, interact and exchange data via the Internet. Gartner estimated the total number of IoT devices to be 8.4 billion in 2017 and predicted an increase to 20 billion by 2020; Intel, meanwhile, projected a growth up to 200 billion by 2020, which equates to nearly 26 smart devices for each human.
The Internet of Health Things (IoHT) is an application of the IoT for health-related purposes, enabling remote patient data collection. These devices range from a simple monitor to a smartwatch and lately into e-textiles; they allow to collect parameters such as activity, sleep, temperature, heart rate, glucose, oxygen and many others, in near real-time, offering a broad range of capability to monitor patients. These devices are playing an increasing role in disease prevention and control, and in managing chronic diseases.
AI is a field of computer science that aims to mimic human intelligence with computer systems. It includes a set of mature technologies that have been evolving for more than 30 years in healthcare and other industries; they include Machine Learning (ML), Natural Language Processing (NLP), Knowledge Base Systems (KBS), and Robotic Process Automation (RPA). In the last 5 years, there has been increased use of ML driven by the introduction of the cloud with high processing power, and increased access to Big Data: the computer learns by detecting patterns in a set of training data and is then able to predict outcomes from newly acquired data. As processing power is being moved with “edge” devices, ML algorithms will increasingly be running from the edge, decreasing the need to exchange the large volume of data needed for ML algorithms. RPA and more specifically bots — such as ALEXA or SIRI —building on speech recognition, cognitive services and machine learning are increasingly part of our daily life; if we customise bots with medical content, they could become very useful medical personal assistants adapted to the context and needs of the patient.
There is a clear intersection between IoT and ML. IoT is about connecting machines that generate large amount of real-time data and making use of these data. AI-Machine Learning (AI-ML) is about learning from data and simulating intelligent behaviour; it needs vast volumes of data to increase their predictive value. By integrating IoT and dedicated AI-ML algorithms, we create a ’connected intelligence’ rather than just connecting technologies. Adding a bot-based personal assistant leverages even further the value of IoT and ML, enabling intelligent and personalised interaction with the patients and creating a ‘smart assistant.’
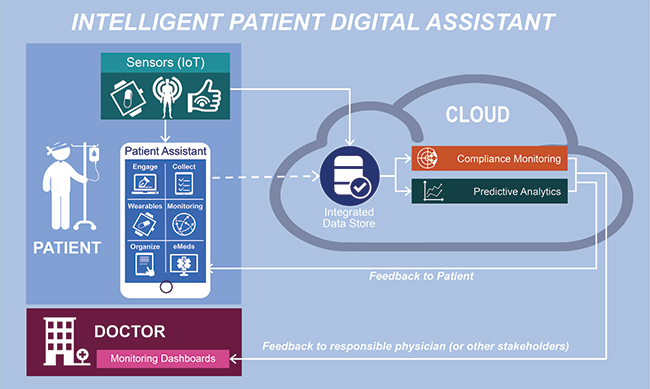
The difficulty with emerging technologies is to understand how to apply them in the real world to maximise their value. In the pharma world, we believe that IoT and AI can best be combined in an Intelligent Patient Digital Assistant (IPA) with the components described below.
The Sensors(IoT) capture information directly from the patient — or from the patient’s home. Usually, data are collected hourly or daily (e.g., glucose, blood pressure) leading to few megabytes (MB) of data. In some cases, like monitoring of patient neurological conditions based on movement, data are collected continuously and can range to several gigabytes (GB)1 per patient per day.
The Patient Personal Assistant combines within a single app all the functions needed to support a patient in his/her daily life. The major difference between the Patient Assistant and a set of integrated apps, is that it builds on AI-Bot technologies to adapt to the patient context and needs; it keeps learning and adapting to the patient preferences as it interacts with him/her and increase the sense of familiarity and ‘caring’. It could include different modules. The Engage module is the core of the Bot, managing patient interactions and informing the patient on his/her disease and on the trials, while keeping him/her engaged in a treatment or in a clinical trial; this module can be extended with eConsent. The Collect module would function as a context-dependent, adaptive electronic Clinical Outcome Assessment/eCOA).The Wearable module displays any specific information directly from the sensors. The Monitoring module manages messages, warning or alert, coming from the predictive AI algorithms; it ensures that the patient understands the message and checks he/she takes the necessary actions, based on the context.
The Patient Personal Assistant could include other optional intelligent modules such as the Organiser to help with scheduling visits to a hospital, book a taxi, order food, call somebody, in the same way an ALEXA or Google Home support people at home. The eMedication module supports the patient in taking medication properly; ideally, it should be linked to a medication dispensing system with Radio Frequency Identification (RFID) technology that allow to confirm if a pill has been taken out of the box or not.
All the information coming from sensors — and potentially other sources such as from the Patient Assistant Collect module — is sent into a centralised integrated data store in the cloud and further analysed. Compliance monitoring checks if a patient is wearing/using the sensors properly by tracking unexpected patterns: for instance, if a patient with muscular dystrophy puts his sensor on his dog, the system will quickly detect that there is an unexpected pattern and will flag an alert. Predictive analytics uses similarl ML to detect specific bad conditions such as risk of heart attack, hypoinsulinemia, asthma crisis, or epilepsy crisis, learning from data from previous patient populations and comparing with the patient data. The algorithm can then generate an alert to the patient directly and/or to the treating physicians or to family.
One major limitation in this model involves the ability to communicate large amount of data needed for the ML algorithm over the net. For instance, activity data collected continuously (10 to 100 times/second) can easily grow to several GBs to be transferred per patient per day. This requires telecommunication capabilities not always available in cities, let alone in rural areas. With edge computing, we can expect the emergence of IoT devices that will be capable to run and maintain ML algorithms locally and only send critical data for audit and archival.
The Intelligent Patient Digital Assistant (IPA) is a ‘remote’ support agent for a patient, providing contextual and adapted information, monitoring patient condition and providing adapted feedback — and potentially health alerts — whenever needed to the patient or to external people, family, treating physician, health care call centre. In clinical research this enables the implementation of patientcentricity in clinical trial execution and opens possibilities for developing a new generation of products, combining drug and technologies.
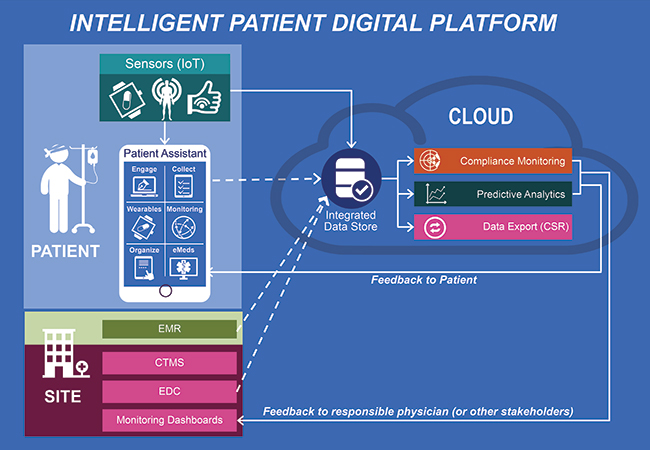
The IPA described above can be extended to support clinical trials by adding sites and investigators with a Clinical Trial Management System (CTMS), integrating data collection from a classical Electronic Data Capture (EDC) system and/or from the EMR and by including data export functions, enabling delivery of a Clinical Study Report (CSR). This extended platform can be used to support new approaches to clinical trial execution, envisioned by pharma for a few years now.
• As the platform allows for ongoing monitoring of the patient, pharma companies may build a case for regulators to put their drug product much faster on the market, under conditional approval.
• By introducing an IPA capable of adapting to the patient context, the platform supports patient-centricity and provides new possibilities to improve patient compliance.
• Thanks to the sensors (IoT), the platform allows for remote monitoring of the patient, a key technology enabler for virtual / remote trials, allowing the patient to remain at home rather than visit the site regularly and, therefore, increasing his/her comfort and readiness to remain in a trial.
• And finally, thanks to the predictive analytics module linked to bot that can regularly adapt advice and/or treatment to the patient specific data and context, personalised medicine becomes affordable at a wider scale.
The other major area of opportunity for pharma is the possibility to develop new products combining digital technologies with drug product to bring a new generation of services to the healthcare market. As an mHealth device, the IPA can be used for patients affected by a chronic disease or elderly people who are at risk of a dangerous clinical events (e.g. crisis of epilepsy or COPD), but do not necessarily require constant support and follow-up. The IPA can collect information and monitor the patient in the background, while at the same time assuring the patient and family that he/she is in ‘safe’ hands. Apple is already tackling this market, coupling the Apple Watch with the iPhone and expanding functionalities to acquire data from both devices, and adding new apps residing on the iPhone. A first application could be the result of their partnership with Janssen2 in Atrial Fibrillation. In this new model, instead of developing drug products as standalone, the industry can start to think how to combine their drug with sensors, bots and additional ML predictive capabilities within an Intelligent Patient Digital Assistant; this helps improve patient safety while also increasing compliance and efficacy — and therefore value — of a treatment delivered just in time to the patient. In addition, such products can save millions of hospitalisation costs, a valuable economy for most countries with ever increasing healthcare cost.
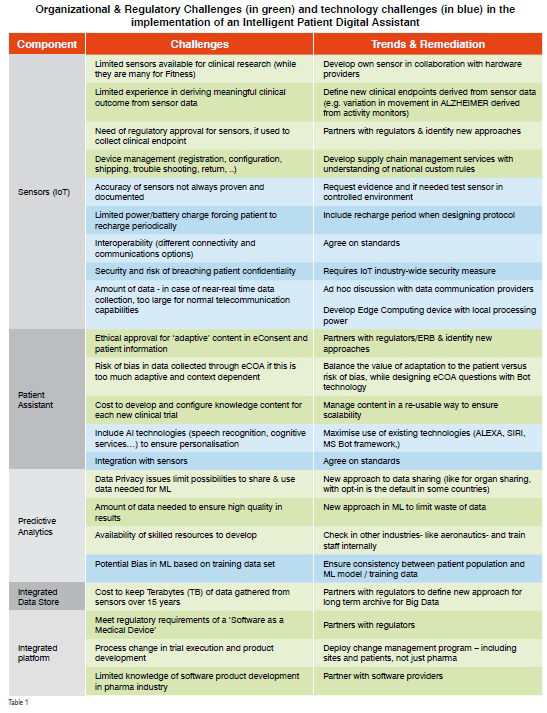
While there are major benefits for the Intelligent Patient Digital Assistant, its implementation will not go without challenges as displayed in the table below. There are organisational, regulatory and technical challenges that will require additional innovation and new ways of thinking, as well as new or adapted regulations.
These aren’t insurmountable challenges. And while it will require hard work and collaboration between many different stakeholders to resolve them, the benefits (patient safety & comfort, increased efficacy with more personalised treatment, decreased cost) far outweigh the effort. We suggest, however, you avoid trying to solve all the problems at once; it is better to start small, find solutions to small problems and bring value step by step.
Conclusions
There is no question that both the healthcare and the pharma markets need new approaches to treatments as the current costs are not sustainable while the patient population is ageing, and chronic diseases are increasing. Digitisation could be a core driver of the change.
Combination of IoT and AI — Machine Learning & Bot — creates ‘smart’ machines simulating intelligent behaviour, making informed decisions with limited human intervention and offering personalised interaction. An Intelligent Patient Digital Assistant as described in this paper can boost productivity of clinical staff, decrease the cost of clinical trial execution and improve patient comfort while keeping (and even improving) quality of care; it is particularly suited to monitor and support treatment of chronic disease and elderly population. And while they are challenges toward implementation at regulatory, organisational and technical levels, they can be overcome with a stepped approach.
One key question is how fast we will be able to introduce these types of platforms in a highly regulated and conservative industry. There is a growing interest in combining IoT and AI across markets: a recent survey3 of 500 IT professionals including 100 top IT executives suggested that “IoT and AI are the most popular technologies currently in use, and top of the list for further investment for businesses seeking increased efficiency and competitive advantage.” The trend applies to life science too with an increasing number of new entrants in digital healthcare with products leveraging IoT and AI. Apple is probably the most aggressive example, but there are many new players coming up every day; they are skilled innovators, flexible, often linked to prestigious universities and supported by venture capital. Not all of them will succeed but the competition is becoming fierce. In a January 2018 report4 on why digital strategies fail, McKinsey mention that “disruption is always dangerous but digital disruptions are happening faster than ever” and they predict that existing business models will be replaced by new digital business models, where “bold movers (attackers and agile incumbent) survive and rise”. Pharma and CROs are the incumbents of clinical research; now is the time for them to be agile and not just think digital but act digital.
References:
1) 1 Terabyte (TB) = 1000 Gigabytes (GB); 1 Gigabyte (GB) = 1000 Megabytes (MB).
• A document with the text included in this paper would be less than 0.5 MB.
•Classical EDC studies would consume 5 to 20 GB per study (based on the number of patients)
2) https://www.biospace.com/article/j-and-j-and-appleteam-up-for-heart-health-program
3) https://sadasystems.com/blog/sada-systems-2018-techtrends-survey
4) https://www.mckinsey.com/business-functions/digitalmckinsey/our-insights/why-digital-strategies-fail