The industry has worked through 2020 to quickly develop a COVID-19 vaccine under pressure. Soon, regulatory activity will shift back to its standard focus, and pharma companies may realise that data integrity standards may have slipped. This article will discuss the importance of data integrity and best practices to follow.
Today, we are in the midst of one of the most challenging periods of modern times. The coronavirus pandemic has engulfed the world, with more than 47.5 million infected and 1.2 million lost lives and those numbers are rising every day. The life sciences and pharmaceutical industry has been at the centre of this storm, working around the clock to evaluate, create, trial and bring to market a wide range of drugs as plausible lines of prevention and treatment for COVID-19.

However, these efforts are haunted by a shortage of resources, restrictions on importing API, social distancing at facilities, disturbed supply chains, and tremendous pressure to quickly manufacture and distribute products. Despite these arduous circumstances, it remains critical for pharma companies to maintain quality and compliance and follow regulatory guidelines. Doing so requires pertinent measures to ensure adherence to Current Good Manufacturing Practice (CGMP) guidelines, and data integrity to meet the requirements of regulators including the U.S. Food and Drug Administration (FDA), the Medicines and Healthcare Products Regulatory Agency (MHRA), and others, in case of an inspection or inquiry.
With normal routines altered in a profound way, what is likely to catch the attention of regulators are the measures taken in regard to the health of the employees, safety protocols and hygiene processes at research and manufacturing facilities. In preparation for these, companies should conduct a rigorous review of policies and procedures to ensure adequate documentation and reporting.
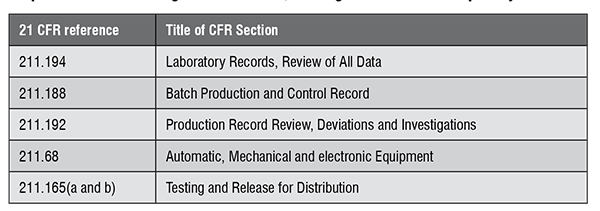
Another area of inspection regulators are likely to focus on is data integrity. Lapses in reliability and accuracy of data — which are essential for ensuring the ‘safety, efficacy and quality of drugs’ — will be closely scrutinised as part of regulatory efforts to protect public health. Electronic data of the analytical equipment and batch records of the products manufactured during this phase are prime examples of data regulators may examine during an inspection or inquiry.
According to regulatory guidelines, data integrity is:
“…the completeness, consistency, and accuracy of data, which should be attributable, legible, contemporaneously recorded, original or a true copy, and accurate.” (FDA)
“…the degree to which data are complete, consistent, accurate, trustworthy and reliable,” and, “Risk-based system design and controls should enable the detection of errors, lapses and omissions of results and data during the data life cycle. Controls may include procedural controls, organizational controls and functional controls.” (WHO)
Poor data integrity practices in the manufacturing and testing of pharmaceuticals products have resulted in a steady rise in the number of enforcement actions, such as warning letters, import alerts and consent decrees. Criticality of data integrity is substantiated by a global report that states that roughly 50 per cent of all Center for Drug Evaluation and Research (CDER) inspection observations (form 483) issued between 2014 and 2018 cite data integrity violations. These violations are even more prevalent in warning letters, with 79 per cent of global drug warning letters during this period citing data integrity issues.
Further, the underlying similarity in the recent warning letters issued by the FDA is to engage a consultant to audit a company’s operations and assist
in meeting FDA requirements for the data integrity remediation activity.
“Your quality system does not adequately ensure the accuracy and integrity of data to support the safety, effectiveness, and quality of the drugs you manufacture… We strongly recommend that you retain a qualified consultant to assist in your remediation. In response to this letter, provide the following…A comprehensive investigation into the extent of the inaccuracies in data records and reporting.”
“We acknowledge that you are using a consultant to audit your operation and assist in meeting FDA requirements. We strongly recommend that you retain a qualified consultant to assist in your remediation… An assessment of the extent of data integrity deficiencies at your facility. Identify omissions, alterations, deletions, record destruction, non-contemporaneous record completion, and other deficiencies. Describe all parts of your facility’s operations in which you discovered data integrity lapses…A comprehensive retrospective evaluation of the nature of the manufacturing data integrity deficiencies. We recommend that a qualified third party with specific expertise in the area where potential breaches were identified should evaluate all data integrity lapses. ”
With the increase in the issuance of warning letters and forms 483 over the years, pharmaceutical companies have become increasingly cognisant of the importance of data integrity to ensure the safety, efficacy and quality of their products. Under the pressures of the COVID-19 pandemic in particular, company leadership should focus on taking proactive measures to manage data integrity risk and ensure compliance with the CGMP regulations before the next FDA inspection happens. These efforts may include review and assessment of procedures as per the guidance of CGMP for responding to COVID-19 infection in employees, to prevent or mitigate potential adverse effects on the safety and quality of drugs from an infected or potentially infected employee engaged in drug manufacturing. Companies should also utilise advanced digital tools and technologies that can proactively identify data integrity lapses in electronic data of certain equipment (e.g. Chromatography Data Systems (CDS), NON-CDS, LIMS, etc.) and highlight on a real-time basis, so management can enable the company to take necessary actions.
Companies that have already received a form 483 and warning letter should consider implementing a remediation plan for the violations identified. This would involve a comprehensive investigation that includes identification of the causes of the lapse, ongoing monitoring and preventive steps for potential recurrence. Since data integrity is a critical component of the basis for maintaining quality and the confidence of regulators, pharmaceutical companies should seek the expertise of a third-party expert to audit company operations and assist in meeting FDA requirements.