This article mainly focuses on the recent advances and applications of Chiral HPLC, LC-MS and SFC applications in Pharmaceutical Analysis.
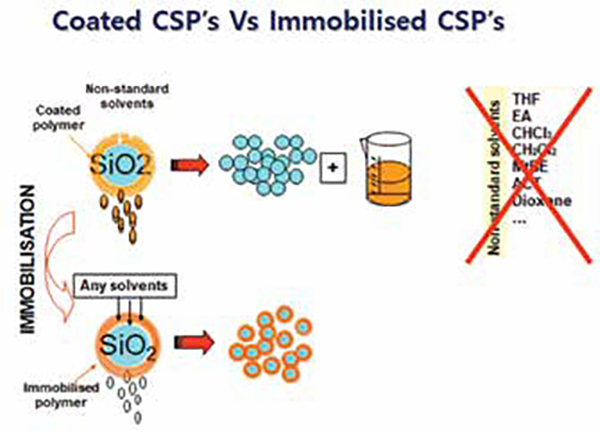
Polysaccharide derivatives are extensively used in chiral stationary phases. They provide multiple advantages including broad enantioselectivity, better resolution ability, easy availability, and high loadability under preparative separation conditions. Commercially available chiral stationary phases (CSPs) of this type are usually coated onto a silica matrix (or) covalently bonded to the silica matrix (immobilized). However, coated CSPs swell or dissolve and finally destroy the enantioselective capacity of the phase with some forbidden organic modifiers. The use of forbidden organic modifiers, such as chloroform, dichloromethane, acetone, ethyl acetate and tetrahydrofuran, in the mobile phase is, therefore, prohibited. To overcome these limitations, new immobilised CSPs were developed, containing polysaccharide derivatives covalently bonded to the silica matrix.
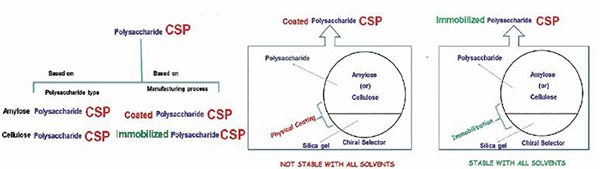
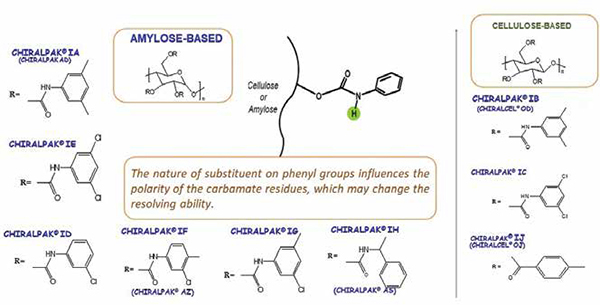
At present, nine immobilised polysaccharide-based CSPs are commercially available Chiralpak IA, IB, IC, ID, IE, IF, IG, IH, IJ. The chemical structures of these selectors are given in the below figure. Different approaches were reported to fix the chiral selector chemically linking the polysaccharide derivatives to the silica matrix, which is considered to be a prerequisite for enantioselective recognition. Consequently, the enantioselective recognition abilities of polysaccharide CSPs may be different. The immobilised CSPs are robust and can be used with a broader variety of solvents, as mentioned above. This extends the application range of these selectors in terms of enantioselectivity, but also in terms of mobile-phase solubility of compounds, offering benefits at both analytical and preparative scales.
Chiral selectors present in amylose and cellulose based CSPs
• Chiral selectors have special types structures (grooves/cavities/baskets)
• Enantiomers become stereo specifically interacted
• The enantiomers is stabilised by various forces…
• Hydrogen bonding, π-π interactions, Dipole induced Diploe attractions etc…
• The combination of these forces is entirely different in each chiral selector
• The enantiomers fit sterogenically into the chiral grooves at different extents
• As a result of these combined effects, the enantiomers elute at different time intervals and mobile phase try to elute them.
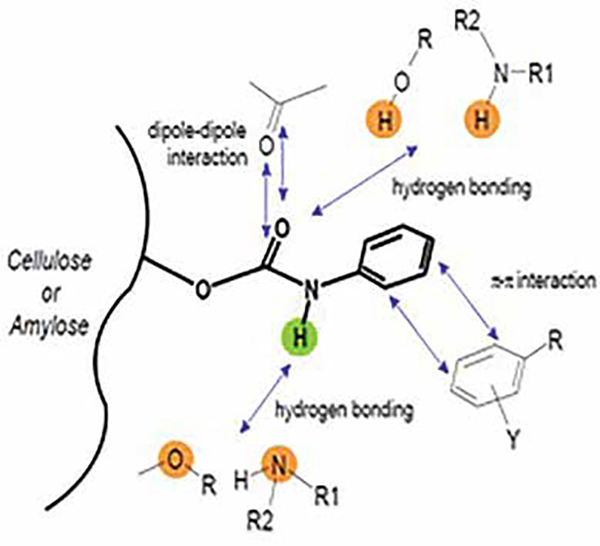
• The success rate on each immobilised stationary phase is some what different for each MP.
• Chiral HPLC is a very versatile technique for the determination of chiral impurities in bulk actives and pharmaceutical formulations.
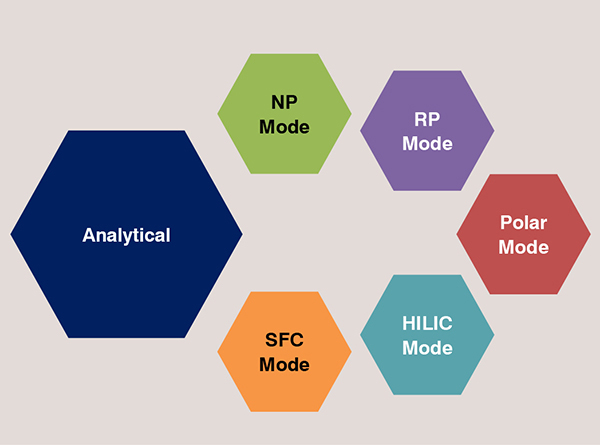
• Using polysaccharide derived CSPs on chiral HPLC, separations can be explored in three common modes: normal phase (NP), polar organic (PO) and reversed phase (RP) chiral LC.
• For both ‘coated’ and ‘immobilised’ CSPs, in NP mode, typically alkane and alcohol mixtures are used as mobile phases.
• In case of ‘immobilised’ CSPs, additionally, solvents such as methyl tertiary butyl ether, ethyl acetate, tetrahydrofuran, dichloromethane, chloroform, 1,4-dioxane etc can be used.
• In PO mode, polar organic solvents such as methanol, ethanol, acetonitrile are used as mobile phase for both ‘coated’ and ‘immobilised’ type CSPs.
• In RP mode, water/buffer in combination with organic solvents such as acetonitrile and methanol are used as mobile phase.
• HILIC mode is suitable for separating polar compounds. Polar samples always show superior solubility in the aqueous mobile phase used in HILIC, which overcomes the disadvantages of the poor solubility often encountered in NP-LC. it can be easily coupled to mass spectrometry (ESI-MS) analysis.
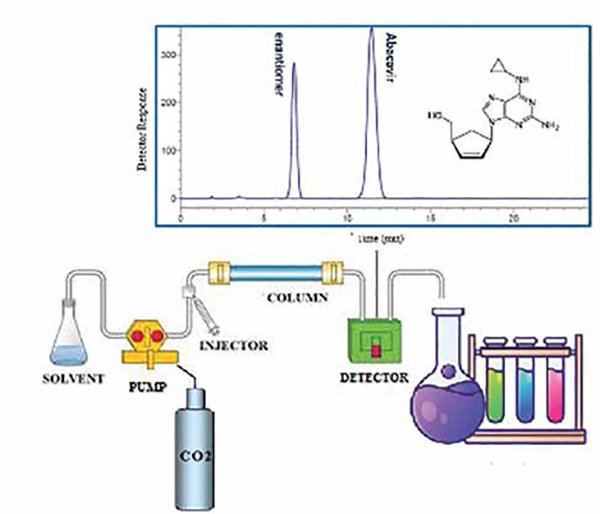
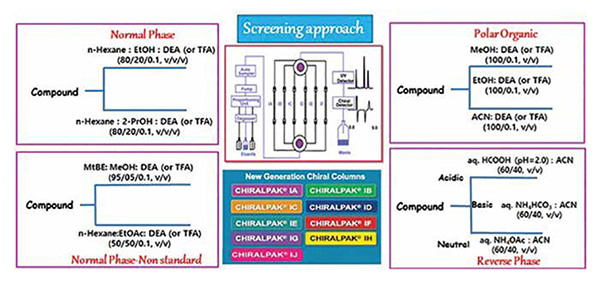
• In view of difficulties in predicting the chiral separation based on the chemical structures, standard screening approach is in practice using HPLC connected with column switching valve as shown in Fig.
Method development-screening approaches
Example: Reversed phase screening and advantages
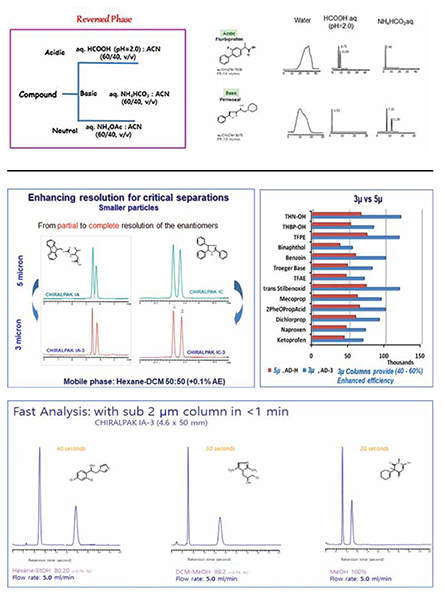
• Better solubility of analytes in salt form
• Less use of toxic solvents
• Compatible for Bio analytical and formulation samples
• Compatible with LC-MS/MS
• Reduce analysis time. So more number of sample analysis posible in shorter times.
• Less efforts in method development as run time is less.
• Cost reduction due to less consumption of mobile pase solvents and time saving.
• Save capital investment and operating costs
• Excellent tool for rapid dvelopments in R&Ds, Drug Discovery research and CRO environments.
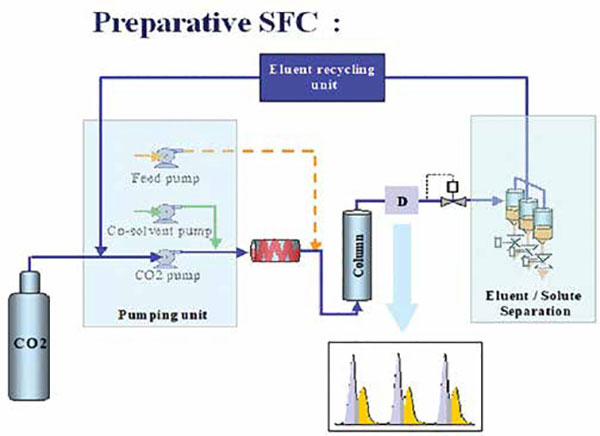
Although SFC has repeatedly proven itself as a performant separation technique for chiral separations, HPLC remains dominant in this field. This is likely due to the limited range of available SFC equipment. However, day to day, the number of reports on chiral SFC applications also increasing in pharmaceutical analysis. Chiral SFC separations can be achieved with high efficiency and minimal mobile phase solvent consumption in a short time span. SFC also has benefits in the context of upscaling to preparative scale, since returning to ambient conditions after analysis evaporates the main eluent (CO2) from the mobile phase. Due to these reasons, interest remains in SFC for enantioseparation of pharmaceutically important compounds and drug substances till now.
When a substance is brought above its critical temperature and pressure, coexisting both phases disappear and a new state called the supercritical fluid is formed.
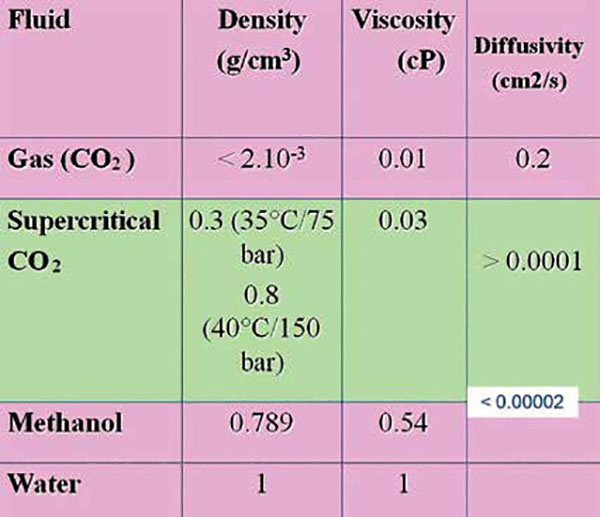
The physical properties of a supercritical fluid are intermediate between those of a typical gas or liquid
• Supercritical fluid is formed if temperature and pressure of a gas or liquid exceed their critical values. CO2 are easily attainable.(TC = 304.12K, pC = 73.74bar)
• CO2 is non-toxic, non-flammable, can be easily purified and is relatively cheap.
• Supercritical fluids have unique features lying between gas and liquid states.
• Liquid-like densities and dissolving capabilities together with gas-like viscosities and diffusion properties make them ideal candidates for major mobile phase components
• Its high molecular diffusivity considerably enhances mass transfer.
• The majority of SFC separations take place in subcritical region due to the addition of organic modifiers
• SFC can substitute both normal phase and reversed phase HPLC separation modes
• Pressure, temperature, mobile phase composition enable the separation of more compounds in reasonable analysis time.
• Wide variety of possible organic modifiers facilitates the optimisation of separation
• The SFC mobile phases enable high flow rates and therefore fast analyses
• Post-analysis evaporation of CO2 keeps products concentrated in the organic modifier
• Better soluble in mixtures of supercritical fluids and organic modifiers than in pure organic solvents.
Eluent system: CO2 (60-95%); Co-solvent: Polar solvents (5-40%) Eg: MeOH, EtOH, IPA, ACN
Elution mode : Isocratic / Gradient
Flow Rates : Typically 3-4 times higher than LC flow
Col Temperature : 10-40°C
Additives : Acidic Analyte: No additive
Neutral Analyte : No additive, Basic Analyte : 0.1-1% DEA / EA / IPA / BA
• Complementary selectivity
• Faster separations and high productivity
• Preparative separations ranging from g to multi Kg scale
• Easy evaporation of fractions
• Lower operating costs and Green technology.