Biomanufacturing is moving toward digital manufacturing with increased application of process analytical technology (PAT) and continuous manufacturing. This article describes experiences in the modelling, design, control, and operation of continuous monoclonal antibody (mAb) manufacturing processes. Fully automated processes employ in-line and at-line PAT that are used to build mechanistic models to confirm process understanding and design real-time feedback control of critical quality attributes (CQAs).
Monoclonal antibodies (mAbs) are the highest selling class of biologics due to their specific action and reduced immunogenicity. As the use of mAbs promotes treating many diseases (e.g., cancer) with better targeted immunological approaches, the development of mAbs will continue to increase.
Process analytical technology (PAT) is increasingly applied in biopharmaceutical manufacturing. PAT is “a system for designing, analysing, and controlling manufacturing through the timely measurements (i.e., during processing) of critical quality and performance attributes of raw and in-process materials and processes, with the goal of ensuring final product quality.” On-line measurements of critical quality attributes (CQAs) lead to increased process understanding and facilitate construction of process models, with most of the PAT being implemented in upstream processing. Standard sensors provide in-reactor analysis of optical density, dissolved oxygen, temperature, and pH. Moreover, online analytics of in-vessel Raman, viable cell density via capacitance, and off-gas and weight control for bioreactor and feed streams can be included. Also, through the use of an autosampler, samples can be sent to at-line cell culture and metabolite analysis.
Another trend is increasing use of continuous operation of biomanufacturing to improve product quality and reduce manufacturing costs. Moving to end-to-end continuous biopharmaceutical manufacturing, from continuous perfusion bioreactors to continuous downstream processes, is also of interest, and several experimental implementations have been reported. The upstream can consist of multiple perfusion bioreactors in parallel, to better balance the flow through the unit operations. Such end-to-end biomanufacturing, when unit operations are tightly integrated, requires that the control strategy is designed to address the propagation of disturbances. The overall process operations can be optimised by using a plant-wide control strategy, such as demonstrated for small-molecule pharmaceuticals.
These trends ultimately lead biomanufacturing towards digital manufacturing, which refers to manufacturing that is centred around a computer system and enhanced by using modern systems engineering tools (e.g., modelling and simulation, process optimisation, and process control) acting in concert. Below are examples of the application of digital manufacturing to continuous bioprocessing.
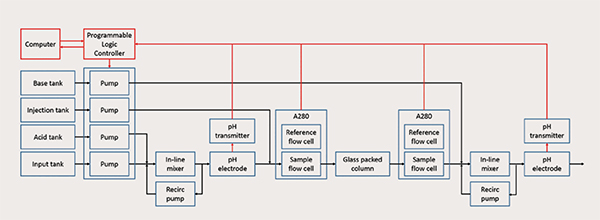
Figure 1: Schematic diagram of the colum-based continuous viral inactivation system
Mechanistic modelling can be developed for all of the unit operations in a continuous biopharmaceutical manufacturing plant. The material balances for the bioreactor are typically modelled as well-mixed, y is the concentration of species of interest in the bioreactor, VL is the liquid medium volume, F is the media flow rate, the subscripts in and out referring to inlet and outlet streams, is the separation factor at the outlet, and r y is the volumetric reaction rate. The separation factor for the cell density is equal to one for fed-batch operation and zero for perfusion operation.
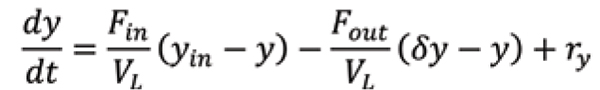
For perfusion operation of Chinese hamster ovary (CHO) cells producing mAbs, differential balances for viable cell density XV and concentrations of substrates S, inhibitors I, and monoclonal antibody P are
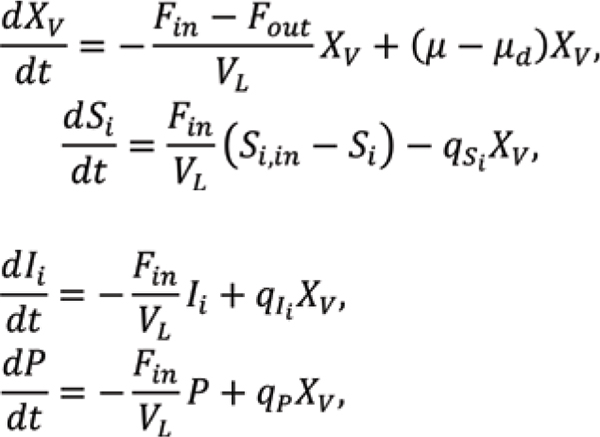
is the specific growth rate, is the specific death rate, qS is the specific substrate consumption rate, qI is the specific inhibitor production rate, and qp is the specific monoclonal antibody production rate. Typically, critical substrates involved are glucose for carbon source and glutamine nitrogen source, and critical inhibitors produced as side products are lactate and ammonia.
For product characterisation, mammalian N-linked glycosylation is important as glycosylation profiles impact activity, immunogenicity, and efficacy of therapies. Glycosylation is a complex biological process beginning from addition of a precursor oligosaccharide in the endoplasmic reticulum (ER) to a series of enzymatic reactions converting the precursor oligosaccharide into a complex carbohydrate in the Golgi apparatus. Many variables have been identified to impact the glycoform distribution including host cell machinery, process conditions, and medium components. For example, important carbon sources such as glucose and galactose impact glycosylation by affecting the nucleotide synthesis. Metal cations facilitate an increase in glycosylation as they act as co-factors for glycosylation enzymes. Metabolic waste products such as ammonia impact culture conditions such as intracellular pH, which in turn affect the glycosylation.
Mechanistic models of glycosylation can contribute to the understanding of these effects and provide a better basis for process design. Glycosylation can be modelled as material balances, considering the Golgi apparatus as a plug flow reactor operating at quasisteady state conditions,
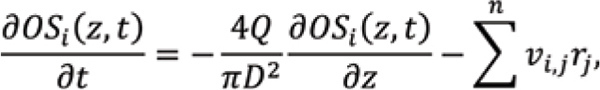
OS is the concentration of oligosaccharide structure, Q is the volumetric flow rate through the Golgi apparatus, D is the diameter of the Golgi apparatus, n is the number of glycosylation reactions, v is stoichiometric coefficient, and r is the reaction rate. The effect of the operating conditions on glycosylation is simulated by the effect of the concentrations of sugar precursors, co-factors, and ammonia on enzyme kinetics. These concentrations are taken as input variables from the aforementioned bioreactor model. Assumed reaction mechanisms can be implemented in the mechanistic model first, and then validated or invalidated by comparisons to experimental data, to improve process understanding.
Continuous tangential flow filtration, chromatography, and diafiltration are commercially available for continuous downstream processing, as described in recent review, but are not commercially available for viral inactivation. To address control challenges with operating the system, we invented a low-cost column-based system (Figure 1) that provides precise control over the pH and residence time, which are critical parameters to ensuring product safety and quality. Model-based pH control is implemented with Bayesian estimation to account for the nonlinearities and variations in the operation. The residence time distribution through the column is periodically estimated during operation as a parametric distribution based on the model through inverse tracer experiments to quantify the minimum residence time and adjust feed flow rates. The system is experimentally demonstrated for tight control of the operating pH, minimum residence time, and logarithmic reduction value over extended operation (Figure 2).
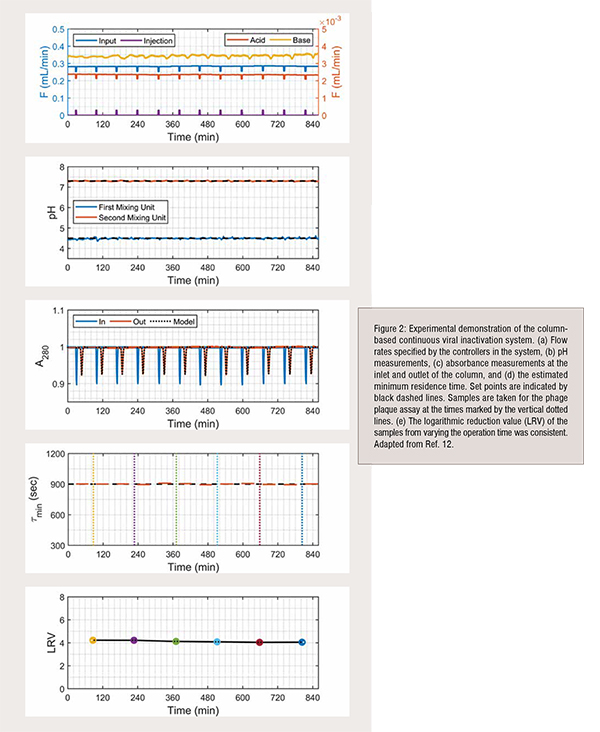
The first stage brings the fluid to a low pH by mixing the input stream with an acidic solution using a mixing unit and an in-line pH electrode. The mixed stream then flows into a column packed with inert glass to incubate at the low pH for a specified residence time for viral inactivation. UV absorbance sensors are placed at the column inlet and outlet to measure the residence time distribution from periodic injections of UV-transparent solution. The pH of the fluid leaving the column is raised by mixing with a basic solution using a second mixing unit and a pH electrode. Programmable logic controller provides low-level control of the system and is connected to a computer to collect data, estimate parameters online, and provide a human-machine interface.
Mechanistic modelling, design, and control are described for the continuous biopharmaceutical manufacturing of mAbs, including the roles of on-line and at-line PAT and basing the designs around modern computer-based tools. Process measurements from PAT are used for development and validation of mathematical models for biopharmaceutical manufacturing processes, to provide insights into multivariable interactions and dynamics from the bioreactor through protein capture, viral inactivation, and polishing. Such plant-wide models enable the design of advanced control strategies for manufacturing products of the highest quality.