Additive Manufacturing (AM), also known as 3D printing, is a rapidly developing technology being explored in various sectors in pharmaceutical applications. The first 3D printed tablet was approved by US Food and Drug Administration (FDA) in 2015, which has created great interest in pharmaceutical 3D printing. This article exhibits the current 3D printing methods, as well as the advantages and disadvantages of this technology in new drug development and pharmacy practice. The challenge and future direction of applying this technology has also been discussed.
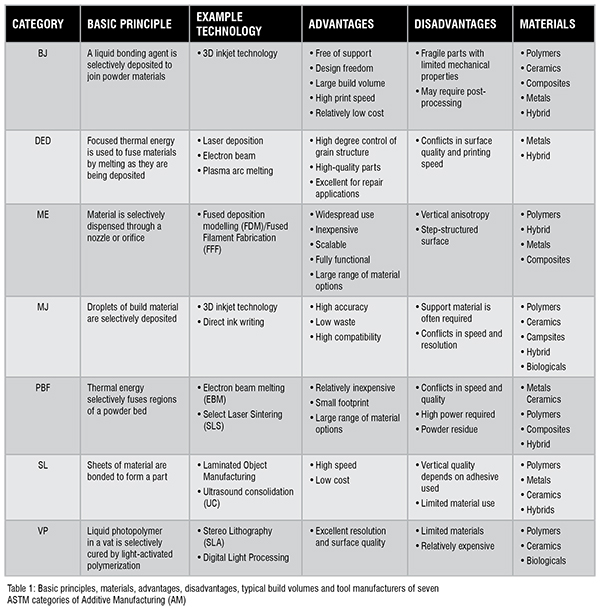
The world’s first 3D printer was invented in the 1980s by using the method of stereolithography (SLA). After that, different types of 3D printers were gradually coming on the scene. The printing processes can be categorised into seven groups, according to the International Organisation for Standardisation (ISO)/ American Society for Testing and Materials (ASTM) 52900:2015 standard classify standard. They are (1) Binder Jetting (BJ); (2) Directed Energy Deposition (DED); (3) Material Extrusion (ME); (4) Material Jetting (MJ); (5) Powder Bed Fusion (PBF); (6) Sheet Lamination (SL) and (7) Vat1 Photopolymerisation (VP).Table 1 shows the basic principles, examples of suitable materials, advantages, and disadvantages of each of these seven systems.
The applications of 3D printing range from the integrated circuit to aviation, clothes to jewellery and foods to medicines. Recently, there has been a rapid rise in applying this new technology for pharmaceutical applications, e.g., to fabricate various dosage forms, such as tablets, capsules, transdermal Microneedle (MN) patches, suppositories, and implants. It has also been used to make novel drug testing systems, replicating complex biological structures in vitro. The selection of 3D printing methods depends on the requirement of printing materials. For pharmaceutical applications, the materials need to be biocompatible, stable, and possess appropriate mechanical strength.
Compared with traditional pharmaceutical manufacturing methods, 3D printing can offer product complexity, personalisation, and on-demand fabrication. First, 3D printing is an enabling tool to construct pharmaceutical products with complex geometries which can confer different drug release kinetics for different conditions. Second, it is useful to fabricate personalised medications, such as personalised dosing which are otherwise challenging to prepare with conventional methods. Third, 3D printing makes it possible for pharmacists fabricate the personalised preparations on the spot and dispense them to the patients. Lastly, 3D printing is cost effective for low-cost production of small quantities of medications, while conventional manufacturing methods are more suitable for mass production.
In this mini review, we summarised the most recent progress in pharmaceutical 3D printing published on academic journals, mainly in 2020.
Rapid prototyping and optimisation of various printing parameters play a vital role in oral dosage forms design. Drug releasing rate from a pharmaceutical preparation could be controlled by selecting suitable material and / or structure for the 3D prints. For instance, instead of the commonly used thermoplastic materials, such as Polylactic Acid (PLA) or acrylonitrile butadiene styrene (ABS), hydrophilic pharmaceutical grade polymers such as Polyvinyl Pyrrolidone (PVP) can be used to print medicine with immediate release properties. Besides, the zero-order drug release profile may be achieved by creating a cylindrical tablet with dense outer regions and porous cores enabling high drug loading.
Clark et al. used an inkjet printer with ultraviolet light to make tablets containing carvedilol which has poor solubility in water. The printing ink consists of carvedilol, poly (ethylene glycol) diacrylate and vinyl-2-pyrrolidone. Tablets of different geometry (thin films, ring, mesh, and cylinder) were printed. It was shown that different release profiles can be obtained by varying the tablet geometry.
In another study, the drug ibuprofen was mixed with cellulose materials to form filaments for 3D printing. Printing parameters, such as shell thickness, layer height, and infill density were varied to print tablets with different properties. The results showed that the tablets can be customised according to the required dose and release rate. It was also demonstrated that Hot-melt Extrusion (HME) in tandem with Fused Deposition Modelling (FDM) printing offer a useful platform for the on-demand drug printing.
In addition to the new dosage forms with customised drug release profiles as mentioned above, AM could also be used to make dosage forms to improve the patients’ experience of taking medications. For example, patients who suffer from dysphagia who have difficulty to take conventional tablets and/or capsules, can take orodispersible film, as an alternative. Orodispersible films are solid oral dosage forms which rapidly dissolve in mouth, which makes them recommended for patients with swallowing problems. Utilisation of water-soluble polymers as a film forming materials are also beneficial to increase drug release, in addition to the printing process itself.
Lastly, tablets with various shapes were printed to cater to patients’ preferences. The printing filaments were prepared by mixing a polymer (hydroxypropyl cellulose), a plasticiser (mannitol) and a lubricant (magnesium stearate). The mixture was extruded using a hot melt extruder to obtain the filaments for subsequent 3D printing using FDM method. Tablets of 10 different shapes were made, namely, disc, torus, sphere, tilted diamond, capsule, pentagon, heart, diamond, triangle and cube. While conventional capsule and disc shapes were acceptable, the torus shape was found to be a preferred novel shape, enabled by the 3D printing method.
Drug delivery through skin is another attractive approach in replace of taking orally. Patches with hundreds of Microneedles (MNs) can penetrate the skin to deliver drugs into the body. Traditional manufacturing of these MN patches is complex and time-consuming. With the 3D printing, the process can be simplified. In addition, by aiding of the 3D scanner and Computer-aided Design (CAD) software, the suitability and comfort can also be improved by tailoring the microneedle patches with specific shapes and sizes.
In one study, the 3D printed MN patches were designed for cisplatin delivery to treat skin tumours. The cisplatin was coated onto the MN shafts by using an inkjet printer. The coated MNs were applied onto skin to suppress tumour growth. In the animal testing, high animal survival rate was observed with the MN patch treatment.
Apart from normal MN patches, MNs with complex design can also be fabricated using 3D printing. For example, Han et al. designed a MN with bioinspired backward-facing curved barbs to enhance the ability for tissue adhesion, for the MN patch to be firmly attached onto skin. This MN patch was fabricated by UV photocuring. The results showed that the pull-out force of the bared MNs was significantly improved than that of non-barbed MNs.
Lastly, MNs can be also printed on a curved surface to fit undulating surfaces. Using a novel photocurable printing ink, Lim et al. fabricated a curved MN patch with varying curvatures to simulate the specific portion of human facial contour using VAT printing method. It was shown that curved MNs complying with facial contours and delivered significantly more drug through skin, to reduce facial wrinkles.
Apart from fabricating dosage forms, 3D printing can also be used to fabricate drug testing systems for new drug discovery applications. For example, transmucosal nasal drug delivery is a useful means to deliver drug into blood circulation. Precise targeting of certain locations within the nasal cavity is important. In one report, a nasal cavity replica was printed using synthetic polymers, to replicate the nasal cavity of a 33-yearold female patent. The nasal replicate was used to assess the spray deposition patterns within the 3D printed nasal cavity model, in order to obtain more efficient nasal sprays.
There are also reports on testing device for ocular drug delivery. For example, a platform was 3D-printed using nylon as the printing materials and was attached to the bottom of 6-well plates with cyanoacrylate glue. The corneal tissue was placed onto the platform for organ culturing in vitro. It was demonstrated that in porcine tissue could maintain the corneal epithelium in the culture system. This organ culture system is potentially useful for ocular drug testing applications.
In another example, a paper-based analytical device was developed to screen herbal medicines for new drug discovery. The identification of bioactive compounds in traditional herbs has become an important approach to obtain novel therapeutic compound. Using the polycaprolactone-chitosan as the printing materials, biological probes were immobilised onto a microfluidic device generated by 3D printing. Then the device was used to screen active compounds in the water extracts of mulberry leaves and lotus leaves. The simple and low-cost device provided a new approach to screen active compounds from natural sources.
While 3D printing has made great progress in pharmaceutical applications, there are challenges hindering the printed products into the market and clinical practice.
First, it is difficult to amend the existing regulatory guidelines and standards to accommodate the 3D printed medicinal products, which are customised fora single subject. It is not feasible to conduct the traditional clinical trials, in testing many subjects. Until now, only limited AM products have been approved by the FDA.
Second, material safety is another concern. There are various materials used for 3D printing, ranging from hydrogels, plastics, ceramics to metal. Their intrinsic characteristics will affect the quality and safety of the printed product. During the printing process, the operating parameters, such as printing temperature, particle size, thermal stability, and mechanical properties also need to be considered. Besides, the choice of 3D printable materials is limited for a certain type of printing, which may limit its application. For instance, the materials used in vat photopolymerisation should have a photocurable functional group. At the same time, the material should also have a safe profile.
Third, cost-effectiveness is another key point to go to market. In general, AM is more cost-effective than traditional manufacturing methods, to make small batches of tablets, capsules, liquids, and/ or customised products. To this end, AM could help to reduce the cost to provide personalised medications to the patients. On the other hand, the use of 3D printing may prevent drug abuse potentially, by means of personalised medicine.
Finally, with the application of the 3D printing technology, the integration of multiple drugs in a single preparation is also possible. It will be useful for the patients who need to take multiple medicines and/or those who have difficulty in recognising various dosage forms of different colour, shape and sizes.
Taken together, the pharmaceutical application of 3D printing is still in its infancy. Based on the current research, 3D printing has great potentials to change the current medical practice, especially in highly customised medications and the way medication is administered to patients. With the rapid development of 3D printing in both the technology itself and the corresponding regulatory framework, it will become one of the indispensable tools in drug development and pharmacy practice in the future.