Current treatment options for asthma, a respiratory condition often stemming from childhood, include beta-2 agonists, inhaled corticosteroids, and leukotriene modifiers. Despite recommendations by the Global Initiative for Asthma, a substantial number of patients are unresponsive to treatment. Paediatric pharmacogenomics could allow for potential preemptive screening, thus improving patient outcomes.
Nearly 300 million people worldwide are affected by asthma, which, left untreated or uncontrolled, decreases quality of life and increases the risk of developing chronic obstructive pulmonary disease (COPD). More than half of these asthma cases originate from childhood. Shortness of breath, tightness, and wheezing are just a few of the symptoms caused by asthma. Although robust evidence supports the efficacy of current treatment options, approximately 100 million additional asthma cases are expected by 2025. Despite recommendations by the Global Initiative for Asthma (GINA) guidelines, up to 70 per cent of patients are still unable to find proper symptom relief. While the lack of adherence and improper diagnosis have been implicated, genetics may also play a role. With widespread intra-variability among medication responses, pharmacogenomics has an emerging place in asthmatic treatment and highlights the importance of optimising a patient’s response to drug therapy while minimising adverse reactions. Although most studies have looked more closely at children, there have been no clinically relevant findings that have warranted specific therapeutic recommendations. Commonly researched medications include beta-2 agonists, inhaled corticosteroids (ICS), and leukotriene modifiers (LTM).
Classified as bronchodilators, beta-2 agonists are categorised into two groups: short-acting beta agonists (SABAs) and long-acting beta agonists (LABAs). Examples of these include albuterol and formoterol, respectively. Commonly utilised in respiratory distress, these agonists induce bronchodilation and relax smooth muscles by binding to the adrenergic receptor located in the lungs. The activation of G-protein coupled receptors (GPCR) causes an increase in cAMP concentration, decreases intracellular calcium and in turn, relieves asthma symptoms (Figure 1).
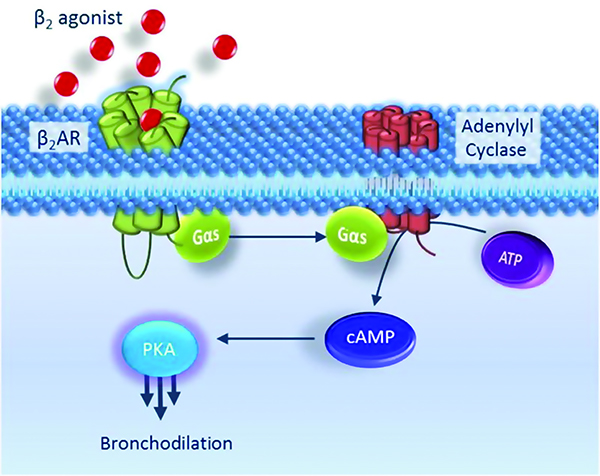
The adrenergic receptor is encoded by the ADRB2 gene and is found on chromosome 5q31-32, an area highly associated with asthma. Position 16 and 27 are two amino acid locations thought to modulate down regulation and airway responsiveness. Choudhry et al. discovered that the presence of Arg at position 16 produced the greatest bronchodilation compared to Gly16Gly when administered albuterol in the Puerto Ricans. Although the study included both Puerto Rican and Mexican children, no genetic association was found among Mexican children. Martinez et al. reported that Arg16Arg and Arg16Gly were more likely to produce greater bronchodilation compared to Gly16Gly when given albuterol. Similar studies by Salah on Egyptian children and Finkelstein on multiethnic children also found favourable results with Arg16Arg in response to albuterol. Both studies found reduced responses in those with Arg16Gly and Gly16Gly genotypes. In contrast, a study conducted by Carroll et al. found that children with the Gly16Gly variation showed a better response, to albuterol, compared to those with the Arg variants. Similarly, Giubergia observed changes in desensitisation over four weeks among some Argentinian children treated with albuterol and reported those with the Arg16Arg genotype displayed a decline in responsiveness to long-term treatment.
Unlike amino acid position 16, conflicting reports in response to SABA therapy at amino acidposition 27 on the receptor have limitedany significant associations. Alghoshaby measured bronchodilator response by FEV1/ forced vital capacity (FVC) and FEV1 whereas Giubergia utilized change in FEV1 over thirty days. While the former found greater improvements with the Gln27Glu variants, the latter found a decrease in responsiveness. A study conducted by Elbahlawanon 31 African American children found that those with the Glu27Glu treated with albuterol needed additional medication for symptom control. These contradictory results provide no clear association at position 27 and bronchodilation in response to SABA.
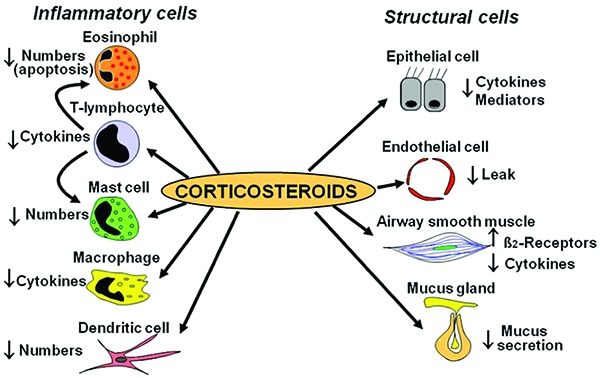
GINA guidelines currently recommend using as needed SABA or ICS -formoterol for children who only require rescue therapy. A low dose of ICS in conjunction with a SABA is recommended as controller therapy for children ages 6-11.ICS work to improve asthma symptoms by decreasing mast cells, cytokines, COX-2, etc thereby decreasing inflammation (Figure 2). FDA indicated to reduce asthma attacks, ICS not only reduce respiratory swelling but also reduce morbidity in both children and adults. Although the risk of systemic exposure is minimal due to their route of administration, side effects such as osteoporosis, glaucoma, and metabolic disturbances are not negligible, especially when used for an extended period (Figure 2).
Tantisira conducted a genome-wide association study (GWAS) and identified the glucocorticoid-induced transcript 1 (GLCCI1) gene and the SNPs, rs37972 and rs37973. Although little is known about GLCCI1, those homozygote for the mutant T allele at SNP, rs37972, yielded a poorer response to ICS compared to those who were heterozygote (CT) or homozygote (CC). Conversely, Vijverberg saw no association with individuals having SNP, rs37972 and ICS response in Northern European children. It was concluded that the mutant T allele was not associated with an increased risk when treated with budesonide. In contrast, Huang found associations in the GLCCI1 gene among Chinese children with SNPs, rs37969 GG, rs37972 CC, and rs37973 AA producing favourable changes measured by maximal mid-expiratory flow.

Variations among cytochrome p450 (CYP) 3A4, 3A5, and 3A7, which are involved in fluticasone metabolism, were assessed in a study conducted by Stockmann involving a group of Caucasian children. CYP3A5 and CYP3A7 were found to have no association, however, CYP3A4*22 children displayed better asthma control due to the reduced activity of this metabolic enzyme, leading to increased therapeutic outcomes. However, this was not seen in a follow-up study of 64 Caucasian children treated with beclomethasone. Observed in this study were CYP3A5 *1/*1, *1/*3, and *3/*3 variations, with the *3/*3 variant displaying the greatest responsiveness based on asthma control scores. It may be recommended that children with the CYP3A4 *22 initiate therapy with fluticasone. Children with CYP3A5 *3/*3 may be advised to avoid beclomethasoneregimens.
Leukotriene modifiers are typically considered when symptoms are uncontrolled by ICS and/or bronchodilators. As seen with previous medications, genetic differences and inter-patient variability also affect a child’s response when given LTM, such as zileuton and montelukast. Although this class may be favoured due to oral administration, nearly half of all children have reported mental status changes like depression and hallucinations. Thus, identifying variants associated with positive LTM response can benefit children who cannot gain symptom control with bronchodilators and ICS.
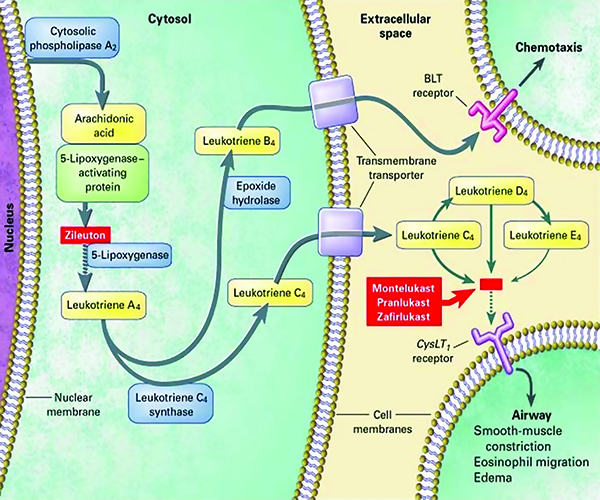
ALOX5 encodes for 5-lipoxygenase (5-LO) and is found on chromosome 10q11.21. 5-LO is involved in the conversion of arachidonic acid into LTA4 and is the rate limiting step in leukotriene synthesis (Figure 3).Klotsman was able to identify five ALOX5 variants associated with montelukastresponsiveness. However, only two of the five variants were found to be significant for favorable outcomes, SNP rs4987105 TT and rs4986832 AA. Conversely, Telleria recognized five copies of the transcription factor binding sequence GGGCGG (rs59439148)in the ALOX5 promoter region as the major allele. Homozygote wild types (5/5) and heterozygotes (5/4) produced favourable responses to montelukast, while the 4/4 repeats produced unfavourable responses. These children demonstrated higher rates of exacerbation and required more doses of their rescue inhaler (Figure 3).
Organic anion transporter polypeptide 2B1 (OATP2B1) are drug transporters responsible for reuptake of various substrates in the liver, intestine, and kidney. Solute carrier organic anion transporter family member 2B1 (SLCO2B1) encodes for these transporters. Mougey found that those with the SNP, rs12422149 GG genotype demonstrated greater improvement at three and six months compared to the those with the AG genotype. Similarly, a study by Li with 50 asthmatic Chinese children displayed lower montelukast clearance if possessing the SLCO2B1 rs12422149 GG genotype, compared to either GA and AA. Due to increased plasma concentrations, children with the GG genotype found better symptom relief when given montelukast. Although CYP2C8 variations were also investigated in this study, no associations with montelukast response were found.
The cost effectiveness and increased quality of life due to pharmacogenomic testing in adults has been clearly demonstrated for those who are genetically inclined to show a reduced response to ICS. Although no research has been conducted in the paediatric population for the same outcomes, identifying children who may also be predisposed to ICS unresponsiveness can encourage providers to initiate LTM therapy sooner. This would eliminate the trial and error of increasing doses of a medication class that the patient will likely fail. Additionally, drug transporters and CYP450 enzymes have potential to guide clinicians when selecting medications.
Unfortunately, limitations in many of these studies include the small sample size, unstandardised outcome measures, and lack of diversity. With recent discussions on race and ethnicity, there is a need for further stratification based on genetic ancestry. Larger paediatric asthmatic studies could improve confidence for the translation of genotype to phenotype, with the common goal of developing the best course of treatment for each individual child. Although preemptive pharmacogenomic testing for asthma is not currently recommended, the hope for universal testing at an early age has the potential to eliminate prolonged medication trial and error.
References:
1. Centers for Disease Control and Prevention. 2019 National Health Interview Survey (NHIS) Data. Available online: https://www.cdc.gov/asthma/nhis/2019/data.htm/ (accessed on 25 October 2021).
2. Dharmage, S.C.; Perret, J.L.; Custovic, A. Epidemiology of Asthma in Children and Adults. Front Pediatr. 2019, 18, 246.
3. Gustafsson, P.M.; Watson, L.; Davis, K.J.; Rabe, K.F. Poor asthma control in children: Evidence from epidemiological surveys and implications for clinical practice. Int. J. Clin. Pract. 2006, 60, 321–334.
4. Billington, C.K.; Penn, R.B.; Hall, I.P. β2 Agonists. Handb. Exp.Pharmacol.2017, 237, 23–40.
5. Carroll, C.L.; Stoltz, P.; Schramm, C.M.; Zucker, A.M. B2-adrenergic receptor polymorphisms affect response to treatment in children with severe asthma exacerbations.Chest2009, 135, 1186–1192.
6. Ortega, V.E.; Meyers, D.A.; Bleecker, E.R. Asthma pharmacogenetics and the development of genetic profiles for personalized medicine.Pharmgenom. Pers. Med. 2015, 8, 9–22.
7. Choudhry, S.; Ung, N.; Avila, P.C.; Ziv, E.; Nazario, S.; Casal, J.; Torres, A.; Gorman, J.D.; Salari, K.; Rodriguez-Santana, J.R.; et al. Pharmacogenetic differences in response to albuterol between Puerto Ricans and Mexicans with asthma. Am. J. Respir. Crit. Care. Med.2005, 171, 563–570.
8. Martinez, F.D.; Graves, P.E.; Baldini, M.; Solomon, S.; Erickson, R. Association between genetic polymorphisms of the beta2-adrenoceptor and response to albuterol in children with and without a history of wheezing. J. Clin. Invest 1997, 100, 3184–3188.
9. Salah, K.; Morsy, S.; Atta, A. Effects of β2-adrenergic receptor polymorphisms on asthma severity and response to salbutamol in Egyptian children.Egypt J. Pediatr. Allergy Immunol. 2012, 10, 81–86.
10. Finkelstein, Y.; Bournissen, F.G.; Hutson, J.R.; Shannon, M. Polymorphism of the ADRB2 gene and response to inhaled beta- agonists in children with asthma: A meta-analysis. J. Asthma2009, 46, 900–905.
11. Giubergia, V.; Gravina, L.P.; Castanos, C.; Chertkoff, L.; Grenoville, M. Influence of beta2-adrenoceptor polymorphisms on the response to chronic use of albuterol in asthmatic children.Pediatr. Pulmonol. 2008, 43, 421–425.
12. Perez-Garcia, J.; Espuela-Ortiz, A.; Lorenzo-Diaz, F.; Pino-Yanes, M. Pharmacogenetics of Pediatric Asthma: Current Perspectives. Pharmgenom. Pers. Med. 2020,
13, 89–103.13. Hikino, K.; Kobayashi, S.; Ota, E.; Mushiroda, T.; Urayama, K.Y.; Kobayashi, T. A meta-analysis of the influence of ADRB2 genetic polymorphisms on albuterol (salbutamol) therapy in patients with asthma. Br. J. Clin. Pharmacol. 2021, 87, 1708–1716.
14. Alghobashy, A.A.; Elsharawy, S.A.; Alkholy, U.M.; Abdalmonem, N.; Abdou, M.A.; Basset, M.A.A.; Pasha, H.F. B2 adrenergic receptor gene polymorphism effect on childhood asthma severity and response to treatment.Pediatr. Res. 2018, 83, 597–605.
15. Elbahlawan, L.; Binaei, S.; Christensen, M.L.; Zhang, Q.; Quasney, M.W.; Dahmer, M.K. β2-Adrenergic receptor polymorphisms in African American children with status asthmaticus.Pediatr. Crit. Care Med. 2006, 7, 15–18.
16. Barnes, P.J. Inhaled Corticosteroids. Pharmaceuticals2010, 3, 514–540.
17. Tantisira, K.G.; Lasky-Su, J.; Harada, M.; Murphy, A.; Litonjua, A.A.; Himes, B.E.; Lange, C.; Lazarus, R.; Sylvia, J.; Klanderman, B.; et al. Genomewide association between GLCCI1 and response to glucocorticoid therapy in asthma. N. Engl. J. Med. 2011, 365, 1173–1183.
18. Vijverberg, S.J.; Tavendale, R.; Leusink, M.; Koenderman, L.; Raaijmakers, J.A.; Postma, D.S.; Koppelman, G.H.; Turner, S.W.; Mukhopadhyay, S.; Palmer, C.N.; et al. Pharmacogenetic analysis of GLCCI1 in three north European pediatric asthma populations with a reported use of inhaled corticosteroids. Pharmacogenomics 2014, 15, 799–806.
19. Huang, J.; Hu, X.; Zheng, X.; Kuang, J.; Liu, C.; Wang, X.; Tang, Y. Effects of STIP1 and GLCCI1 polymorphisms on the risk of childhood asthma and inhaled corticosteroid response in Chinese asthmatic children. BMC Pulm. Med. 2020, 20, 303.
20. Stockmann, C.; Fassl, B.; Gaedigk, R.; Nkoy, F.; Uchida, D.A.; Monson, S.; Reilly, C.A.; Leeder, J.S.; Yost, G.S.; Ward, R.M. Fluticasone propionate pharmacogenetics: CYP3A4*22 polymorphism and pediatric asthma control. J. Pediatr. 2013, 162, 1222–1227, 1227.e1–2.
21. Stockmann, C.; Reilly, C.A.; Fassl, B.; Gaedigk, R.; Nkoy, F.; Stone, B.; Roberts, J.K.; Uchida, D.A.; Leeder, J.S.; Sherwin, C.M.; et al. Effect of CYP3A5*3 on asthma control among children treated with inhaled beclomethasone. J. Allergy Clin. Immunol. 2015, 136, 505–507.
22. Drazen, J.M.; Israel, E.; O’Byrne, P.M. Treatment of asthma with drugs modifying the leukotriene pathway. N. Engl. J. Med. 1999, 340, 197–206.
23. Choi, J. Leukotriene Receptor Antagonists. Available online: https://www.ncbi.nlm.nih.gov/books/NBK554445/ (accessed on 5 November 2021).
24. Mougey, E.; Lang, J.E.; Allayee, H.; Teague, W.G.; Dozor, A.J.; Wise, R.A.; Lima, J.J. ALOX5 polymorphism associates with increased leukotriene production and reduced lung function and asthma control in children with poorly controlled asthma.Clin. Exp. Allergy 2013, 43, 512–520.
25. Klotsman, M.; York, T.P.; Pillai, S.G.; Vargas-Irwin, C.; Sharma, S.S.; van den Oord, E.J.; ,Anderson, W.H. Pharmacogenetics of the 5-lipoxygenase biosynthetic pathway and variable clinical response to montelukast.Pharmacogenet. Genom. 2007, 17, 189–196.
26. Telleria, J.J.; Blanco-Quiros, A.; Varillas, D.; Armentia, A.; Fernandez-Carvajal, I.; Jesus Alonso, M.; Diez, I. ALOX5 promoter genotype and response to montelukast in moderate persistent asthma.Respir. Med. 2008, 102, 857–861.
27. Mougey, E.B.; Feng, H.; Castro, M.; Irvin, C.G.; Lima, J.J. Absorption of montelukast is transporter mediated: A common variant of OATP2B1 is associated with reduced plasma concentrations and poor response.Pharmacogenet. Genom. 2009, 19, 129–138.
28. Li, Q.; Wang, K.; Shi, H.Y.; Wu, Y.E.; Zhou, Y.; Kan, M.; Zheng, Y.; Hao, G.X.; Yang, X.M.; Yang, Y.L. Developmental Pharmacogenetics of SLCO2B1 on Montelukast Pharmacokinetics in Chinese Children. Drug Des. Devel. Ther. 2019, 13, 4405–4411.
29. Wu, A.C.; Gay, C.; Rhett, M.D.; Stout, N.; Weiss, S.T.; Fuhlbrigge, A.L. Pharmacogenomic test that predicts response to inhaled corticosteroids in adults with asthma likely to be cost-saving. Pharmacogenomics 2015, 16, 591–600.