Although conventional freeze drying in vials is still the predominant format, lyophilisation in alternative formats, such as pre-filled syringes, and alternate technologies such as spray freeze drying are becoming more popular.
Lyophilisation or freeze drying is a process widely used in the preparation of biopharmaceuticals and biologicals (Rey & May 2004) because it allows greater storage stability for otherwise labile biomolecules, provides a convenient storage and shippage format and following reconstitution rapidly delivers the product in its original formulation, ready for use.
The worldwide biopharmaceuticals market may well be worth US$ 40 billion annually and comprises over 100 licensed products with many more in the development pipeline. A large proportion of these products are lyophilised and whereas this includes more traditional products such as coagulation factors, cellular-derived vaccines and immunoglobulins, it also is a popular format for the products of the biotechnology industry, monoclonal antibodies growth factors, cytokines and recombinant vaccines (Constantino & Pikal 2004). Although conventional freeze drying in vials is still the predominant format, lyophilisation in alternative formats, such as pre-filled syringes (Hottot et al. 2009), and alternate technologies such as spray freeze drying especially where inhaled formats are envisaged (Misra et al. 2009) are becoming more popular.
Lyophilisation as a commercial process has been in existence for over 50 years and it was once a poorly understood technology, often thought to be more art than science. It is now increasingly guided by developmental design, based on information derived from analytical studies and optimised by means of high technology process monitoring methods.
Recent trends – Thermal methods
In lyophilisation a usually aqueous solution of the active component is dried at sub-ambient temperatures, removing the majority of the constituent water but maintaining the biological activity. Lyophilisation is composed of three basic stages preceded by a number of enabling technologies. A product is firstly formulated, sterile filtered (at least for therapeutic applications) and dispensed into appropriate containers. It is then transferred to the freeze dryer and the process begun by a freezing step, then a period of sublimation under vacuum at sub-ambient temperatures, and finally a further drying period at ambient or elevated temperatures to deliver a product of suitable residual water content. At this point, the atmospheric headspace pressure is adjusted according to process and the containers sealed by stoppering down of the halobutyl or similar closures. The product is then removed for crimping or sealing, labeling and assessment, for compliance with pre-defined quality attributes, before release.
In order to maintain a homogeneous and robust macroscopic appearance in the dried state both the process conditions and formulation should be appropriate. By lowering the temperature of the sample the majority of the water is crystallized as ice, any crystallisable salts may have crystallised and the biologically active material is trapped in a glassy state where the biological material, together with excipient molecules and associated water become effectively immobilised, this is termed the glass transition point. Only at this point can drying begin, if the macroscopic structure of the freeze drying material is to be maintained. If this temperature is exceeded then collapse results and this collapsed state may trap higher levels of residual water and possibly result in reduced stability, though the generality of this concept has been challenged by some recent examples (Wang et al. 2004). It is possible to prevent macroscopic collapse and to deliver an acceptable appearance by generating a crystalline structure using excipients such as mannitol or glycine, but if the biological material is trapped in a collapsed amorphous state surrounded by this structure there may still be impaired stability (Passot et al. 2007). This may be similar to the phenomenon described by some workers as micro-collapse where some shrinkage results but the overall structure is maintained.
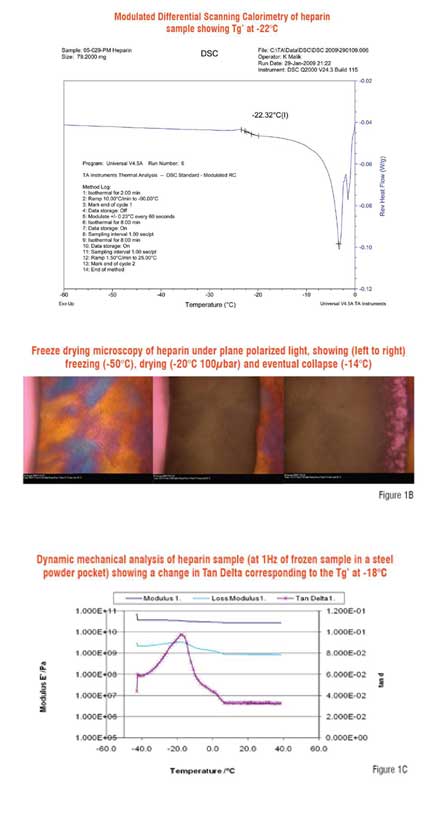
The determination then of the glass transition temperature is very important and there now exist a number of powerful techniques for its determination (Kettet al. 2005). Historically, eutectic or electrical resistivity monitors were developed, which were simple to use and gave broad indication of the gross transition temperature, though these techniques were less appropriate where mainly non-ionic formulations were used. More powerful techniques such as modulated differential scanning calorimetry can identify glass transitions but these must be well separated from the energetically far larger eutectic or melt events for them to be adequately resolved. Other techniques such as dynamic mechanical analysis, which are less influenced by the melt of ice, may give useful information, as may techniques such as electrical impedence (Rey in Rey & May 2004, Ward & Matejtschuk 2007). Freeze drying microscopy, though it is not a thermal analysis method as such, is an extremely valuable tool in determining the temperature at which collapse is observed (Fleck et al. 2003). Samples of volume as little as 2-5 ?L may generate useful data as the freezing, thermal tempering and finally sublimation are modelled at a microscopic scale. Figure 1 gives examples for heparin, comparing some of these techniques. The comparison of glass transition and collapse temperature data and their interpretation has been recently discussed (Meister & Geiseler 2008)
Recent trends – PAT
Once such temperatures are established, pilot trial studies can be performed in laboratory-scale freeze dryers and the cycle to be used designed based on the results obtained and allow for formulations to be compared and biological activity and its stability assessed (Matejtschuk et al. 2009).The scale up from these laboratory studies to production scale is however a challenge and one which requires an understanding not only of the physical properties of the material to be dried (Nail et al. 2002), but also the influence of the container and the freeze dryer on the drying process (Geiseler & Lee 2008).
This issue is increasing being addressed by Process AnalyticalTechnology (PAT) where a combination of non-invasive sensory methods are allowing crucial parameters such as the shelf temperature, vacuum applied and length of primary drying and secondary drying to be determined. Once, such parameters had to be studied by series of pilot scale trials, followed by post-run Quality Control analysis. Now the information gained by non-invasive monitoring can streamline the scale up process and even be used in real time to define the run cycle used. Among these monitoring techniques are manometric pressure rise testing (Geiseler et al. 2007a), changes in water vapour flux generation (using laser light absorption in the spool inter connecting chamber and condenser (Geiseler et al. 2007b) and cold plasma desorption (Mayersese et al. 2007) which look at the overall sublimative properties of a batch. There are other techniques based on gravimetric methods which measure mass loss over primary drying in individual vials (Schmid and Geiseler 2008) and the use of non invasive NIR and Raman Spectroscopy (De Beer et al. 2009) to study water loss. Some of these technologies are applicable only in laboratory or pilot scale equipment whereas others can be fitted in production scale machines also.
Lyophilisation can result in the loss of biological activity, thought to be due often to the effects of dehydration and stripping away of the water associated with the three dimensional structure of a biopharmaceutical drug. For this reason water-replacing excipients such as non-reducing sugars are particularly good lyoprotectants though whether this is due to their ability to replace water in the dried state (Allison et al. 1999) or to form a rigid glass in which the biologically active material is immobilised (Franks et al. 1991), or a combination of both mechanisms, is still a matter of research .
Certain materials may require specific stabilisers, others may benefit from a combination of stabilisers (Hubbard et al. 2007, Amorij et al. 2007). Proteins are often themselves excellent stabilisers and combinations of polymeric and small molecule stabilisers may be preferable in achieving the long term stability of biopharmaceuticals (Wang 2000). Recent studies have investigated the impact of cycle design on long term stability (Luthra et al. 2008) and the importance not just of the glass transition but of dynamic mobility within the lyophilised material on long term stability (Pikal et al. 2008).
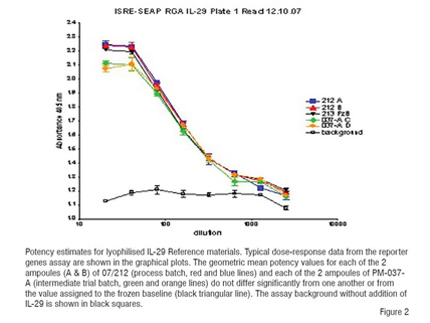
The rise of interest in biosimilar or biogeneric drugs in the biopharmaceutical industry requires reliable, internationally recognised, stable and well-characterised biological reference materials to act as controls in development and regulation. These are particularly valuable where the activity of the biological product requires characterisation against both bioassays and biophysical means (Silva et al. 2008). Such reference materials must be more stable than the drugs they are compared with and so lyophilisation to exacting quality control parameters is necessary (WHO 2006). By comparison of therapeutic materials with these standards, often across a range of assay methods, better understanding of dosage and biological equivalence can be achieved and this is complementary to the extensive biophysical and molecular characterisation which is performed. Whereas once such reference materials were usually sera or plasma now a whole range of purified as well as part-purified materials may be used and in addition to proteins, DNA, RNA and even cellular materials are being prepared as standards. To meet their specification these materials like the products they characterise are lyophilised to ensure stability and consistency. A comparison of the potencies of development and definitive batches of interleukin-29 reference material are shown in Figure 2 using a cell-based assay. The reactivity of the trial and definitive batches closely paralleled each other and the frozen unlyophilised material.
Lyophilisation is progressively becoming a more rationally controlled process . With the rise of better analytical technologies, to understand the physicochemical processes of freezing and sublimation, together with a range of process analytical controls to optimise the freeze drying cycles and scale them for manufacture, the technology can be applied with confidence to high value biotechnology products. By use of high order lyophilised reference materials the characterisation of new vaccine and biotechnological products, both novel and biosimilars, can be better assured and the process of developing and controlling biopharmaceutical medicines facilitated.
The author thanks his NIBSC colleagues Dr Tony Meager and Kiran Malik for use of their data and John Gearing (Gearing Scientific Ltd, Ashwell,UK) for the DMA analysis.
References:
Rey, L & May, JC (2004) eds. “Freeze drying/Lyophilization of Pharmaceutical and Biological Products” Marcel Dekker, New York.
Constantino HR & Pikal, MJ eds. (2004) Lyophilization of Biopharmaceuticals, AAPS Press, Arlington, VA, USA..
Hottot A, Andrieu J, Hoang V, Shalaev EY, Gatlin LA, Ricketts S (2009) Experimental study and modeling of freeze drying in syringe configuration. Part II : Mass and heat transfer parameters and sublimation end points. Drying Technol 27;49-58.
Misra A, Jinturkar K, Patel D, Lalani J, Chougule M (2009) Recent advances in liposomal dry powder formulations: preparation and evaluation. Expert Opin. Drug Deliv. 6;71-89.
Wang DQ , Hey JM, Nail SL (2004) Effect of collapse on the stability of freeze dried recombinant Factor VIII and alpha-amylase. J Pharm Sci 93(5);1253-63
Passot S, Fonseca F, Barbouche N, Marin M, Alarcon-Lorca M, Rolland D, Rapaud M. (2007). Effect of product temperature during primary drying on the long-term stability of lyophilized proteins. Pharm Dev Technol 12:543–553.
Kett,V.,McMahon,D., Ward,K. (2005) Thermoanalytical techniques for the investigation of the freeze drying process and freeze-dried products. Curr.Pharm. Biotechnol.,6 (3), 239-250.
Ward K & Matejtschuk P (2007) The use of Impedance Analysis for the study of critical events in the frozen state for formulation and lyo-cycle development. In “New Ventures in Freeze Drying” IIF-IIR, Paris, France. ISBN 978-2-913149-60-1
Fleck, R,A,, Green, E., Matejtschuk,P. (2003) Freeze drying microscopy. G.I.T. Imaging & Microscopy 5;11-14.
Meister, E., Giesler, H (2008) A significant comparison between collapse and glass transition temperatures. Eur. Pharm. Rev. 13(5), 73-79
Matejtschuk ,P., Malik ,K., Tierney, R., Sloth Wilhelmsen, E., Preneta-Blanc, R., Rigsby, P., Sesardic, D.(2009)Optimization of the formulation for a candidate lyophilised tetanus toxoid reference preparation. Biologicals 37 (2009) 1-7
Nail SL, Jiang S, Chongprasert S, Knopp SA.(2002). Fundamentals of freeze-drying. Pharm Biotechnol. 14; 281-360
Geiseler H., Lee G (2008) Effects of vial packing density on drying rate during freeze drying of carbohydrates or a model protein measured using a vial weighing technique. Pharm Res 25; 302-12.
Geiseler H, Kramer T, Pikal MJ (2007a) Use of manometric temperature measurement and SMART freeze dryer technology for development of an optimised freeze-drying cycle. J Pharm Sci 96;3402-18.
Geiseler H, Kessler WJ, Finson M, Davis SJ, Mulhall PA, Boss V, Debo DJ, Pikal MJ (2007b) Evaluation of tunable diode laser absorption spectroscopy for in process water vapour mass flux measurements during freeze drying. J Pharm Sci 96; 1776-93.
Mayeresse Y, Veillon R, Sibille PH, Nomine C (2007) Freeze drying process monitoring using a cold plasma ionization device . PDA J Pharm Sci Technol 61;160-74.
Schmid S , Gieseler H. (2008) Evaluation of a new wireless temperature remote interrogation system (TEMPRIS) to measure product temperature during freeze drying. AAPS Pharm Sci Tech 9; 729-39.
De Beer TRM , Vercruysse P, Burggraeve A, Quinten T, Ouyang J, Zhang X, Vervaet C, Remon JP, Baeyens WRG (2009) In-line and real-time process monitoring of a freeze drying process using Raman and NIR Spectroscopy as complementary Process Analytical Technology tools. J Pharm Sci. in press. Doi10.1002/jps.21633
Allison SD, Chang B, Randolph TW, Carpenter JF.(1999) Hydrogen bonding between sugar and protein is responsible for inhibition of dehydration-induced protein unfolding. Arch Biochem Biophys 365;289-98
Franks F Hatley RMH, Matthias F( 1991) Materials science and the production of shelf-stable biologicals . BioPharm 4;38
Hubbard AR, Bevan S, Matejtschuk P (2007) Impact of residual moisture and formulation on Factor VIII and Factor V recovery in lyophilized plasma reference materials. Anal Bioanal Chem 387; 2503-7.
Amorij J-P, Meulenaar J, Hinrichs WLJ, Stegmann T, Huckriede A, Coenen F, Frijlink HW (2007) Rational design of an influenza subunit vaccine powder with sugar glass technology: preventing conformational changes of haemagglutinin during freezing and freeze-drying. Vaccine 25; 6447-57.
Wang W (2000) Lyophilization and the development of solid protein pharmaceuticals. Int J Pharm 203;1-60.
Luthra SA, Hodge IM, Utz M, Pikal MJ ( 2008) Correlation of annealing with chemical stability in lyophilized pharmaceutical glasses J Pharm Sci 97(12) 5240-51
Pikal MJ, Rigsbee D, Roy ML, Galreath D, Kovach KJ, Wang B, Carpenter JF, Cicerone MT(2008) Solid state chemistry of proteins: II. The correlation of storage stability of freeze-dried human growth hormone (hGH) with structure and dynamics in the glassy solid. J Pharm Sci. 97(12):5106-21.
Silva MM, Lamarre B, Cerasoli E, Rakowska P, Hills A, Bailey MJ, Wheeler JX, Burns CJ, Gaines-Das RE, Jones C, (2008) Physicochemical and biological assays for quality control of biopharmaceuticals: interferon alpha-2 case study. Biologicals 36(6);383-92
WHO (2006) Recommendations for the Preparation, Characterisation and Establishment of International and other Biological Reference Standards. WHO Tech Report series 932 Annex II.